Summary
Along with an increase in obesity and insulin resistance, there has been an increase in the use of concentrated insulin. This article discusses new products and how they will be incorporated into clinical practice, beneficial alternative systems, whether new insulin formulations are meeting these expectations, as well as guidelines and experience with the long-acting insulin analogs in pregnancy.
- Obesity Diabetes Mellitus
- Insulin
- Hyperglycemia/Hypoglycemia
- Obesity
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes Mellitus
- Insulin
- Hyperglycemia/Hypoglycemia
Along with an increase in obesity and insulin resistance, there has been an increase in the use of concentrated insulin. Mary Korytkowski, MD, University of Pittsburgh, Pittsburgh, Pennsylvania, USA, discussed these new products and how they will be incorporated into clinical practice.
More concentrated forms of insulin (U-500 contains 500 units of regular insulin in each mL of product) prolong absorption and extend duration of action. Four types of patients are candidates for U-500 insulin: those with severe insulin resistance (defined as requiring >200 units of U-100 insulin per day or >2 units/kg/day), those with type A or type B insulin resistance syndrome, and women with gestational diabetes with severe insulin resistance. In patients with insulin-resistant diabetes who require higher concentrations of insulin, the use of U-500 regular insulin provides the same or better glucose control compared with U-100 insulin, with fewer daily injections and women with gestational diabetes with severe insulin resistance. In patients with insulin-resistant diabetes who require higher concentrations of insulin, the use of U-500 regular insulin provides the same or better glucose control compared with U-100 insulin, with fewer daily injections and reduced injection volume [Quinn SL et al. Pharmacotherapy 2011].
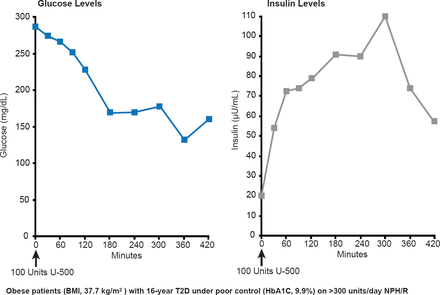
Pharmacokinetic studies have shown that uncontrolled severely insulin-resistant type 2 diabetes mellitus (T2DM) in obese patients can be controlled with U-500 regular insulin. Insulin concentrations rise briskly within 30 minutes and remain elevated for at least 7 hours. Glucose levels stabilize at ∼150 mg/dL after 3 hours (Figure 1) [Davidson MB et al. Diabetes Care 2010].
Use of U-500 Insulin in Obese Patients With Type 2 Diabetes
BMI=body mass index; T2D=type 2 diabetes;
Reproduced from Davidson MB et al. U-500 Regular Insulin: Clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care 2010;33(2):281–283. With permission from the American Diabetes Association.
The use of U-500 alone or as part of combination therapy with rapid- or long-acting insulin significantly (p<0.02) reduced HbA1C, increased weight and increased total daily insulin dose in patients with T2DM [Lowery JB et al. Diabetes Technol Ther 2012].
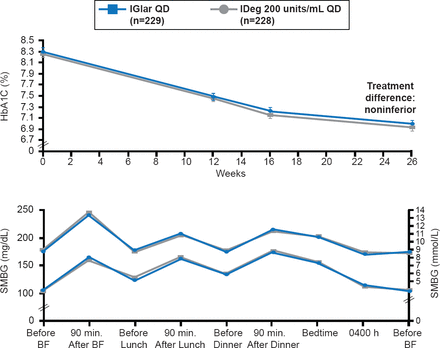
As the obesity epidemic has grown, so has the prevalence of insulin resistance. As a result, the number of patients who require large amounts of U-100 insulin to achieve desired levels of glycemic control has increased. To minimize the volume of insulin necessary to achieve glycemic control, many patients have been switched to U-500 since it will provide comparable insulin doses at one fifth the volume. However, overdosing on U-500 and subsequent hypoglycemia has prompted the development of new concentrated insulin preparations, such as degludec (U-200) and glargine (U-300). Both drugs have been shown to have a prolonged duration of action without an increase low risk of hypoglycemia in patients with type 1 diabetes (T1D) and insulin-naïve patients with T2DM [Gough SCL et al. Diabetes Care 2013]. Glycemic control was similar with both drugs, with a low risk of hypoglycemia (Figure 2).
Effect of Degludec U200 Versus Glargine U100 on Glucose Levels
IDeg=insulin degludec; IGlar=insulin glargine; SMBG=self-monitoring of blood glucose.
Reproduced from Gough SCL et al. Low-Volume Insulin Degludec 200 units/mL Once Daily Improves Glycemic Control Similar to Insulin Glargine With a Low Risk of Hypoglycemia in Insulin-Naïve Patients With Type 2 Diabetes: A 26-week, randomized, controlled, multinational, treat-to-target trial: The BEGIN LOW VOLUME trial. Diabetes Care 2013; doi:10.2337/dc12-2329. With permission from the American Diabetes Association.
Fear of insulin injections is a real barrier that prevents some patients from achieving optimal glycemic control. To overcome this barrier, investigators have focused on developing new insulin delivery systems. William Cefalu, MD, Pennington Biomedical Research Center, Baton Rouge, Louisiana, USA, discussed a number of alternative systems that may have the potential to increase compliance, provide better metabolic control, and improve quality of life.
Proposed systems include a microneedle model that would breach the stratum corneum barrier to facilitate effective transport of molecules across the skin and the use of ultrasound for transdermal insulin delivery. In development is a buccal insulin (Oral-lyn formulation), which is human rDNA insulin (regular) dissolved in a buffer at neutral pH and administered as a aerosolized, aqueous formulation. Medical acceptability and commercial success require that the formulation be modified to achieve an ideal insulin dose with only 2 to 4 sprays.
Oral insulin offers the opportunity to mimic physiological insulin release, but work remains to promote bioavailability. In a Phase 2 study, oral insulin capsules in conjunction with subcutaneous insulin injections were well tolerated and effectively reduced glycemia throughout the day in uncontrolled T1D [Eldor R et al. PLoS One 2013].
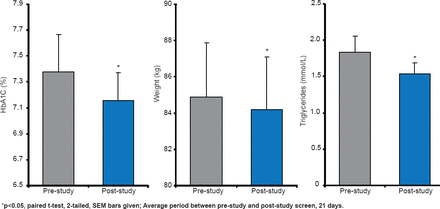
The Axcess Oral Drug Delivery System uses enteric-coated capsules to protect insulin in its passage through the stomach. A Phase 2 trial reported significant (p<0.05) lowering of HbA1C, weight, and triglycerides in patients with T2DM (Figure 3) [Luzio SD et al. Diabetes Obes Metab 2010].
Glucose-Lowering Effect of Capsulin in T2DM
Reproduced from Luzio SD et al. The glucose lowering effect of an oral insulin (Capsulin) during an isoglycaemic clamp study in persons with type 2 diabetes. Diabetes Obes Metab 2010;12(1)82–87. With permission from John Wiley and Sons.
Another approach under development is the use of hepatic directed vesicles that target hepatocytes and restores the liver's key homeostatic function of storage and release of glucose. In closing, Dr. Cefalu discussed inhaled insulin by which 2- to 5-μm particles are disbursed throughout the lung where the physiological pH causes a rapid dissolution of the particles into free insulin and fumaryl diketopiperazine (FDKP), both of which are rapidly absorbed into the blood stream. FDKP acts as the delivery system.
The ideal insulin should attain near normoglycemia, produce very low rates of hypoglycemia, provide consistent glucose levels after injection, and minimize weight gain. Tom Donner, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA, discussed whether the new insulin formulations are meeting these expectations.
Intensive treatment is still associated with excessive hypoglycemia in adults and children. Degludec is a new, ultra-long-acting basal insulin with a 25-hour half-life and >42-hour duration of action. It has similar HbA1C-lowering efficacy when dosed at a fixed time or at intervals of 8 to 40 hours [Meneghini L et al. Diabetes Care 2013]. Insulins degludec and glargine produce similar long-term glycemic control in insulin-naïve patients with T2DM, but degludec has lower rates of nocturnal hypoglycemia [Zinman B et al. Diabetes Care 2012].
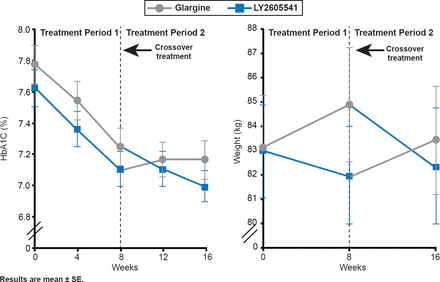
LY2605541 modified with a 20-kDa polyethylene glycol moiety is a new, long-acting basal insulin. Its larger size delays absorption and slows clearance. In patients with T2DM, LY2605541 and glargine showed comparable glucose control and total hypoglycemia rates, but LY2605541 had reduced intraday variability, lower nocturnal hypoglycemia, and weight loss relative to glargine [Bergenstal RM et al. Diabetes Care 2012]. In patients with T1D, LY2605541 demonstrated greater improvements in glycemic control, increased total hypoglycemia, and reduced nocturnal hypoglycemia, as well as reduced weight and lowered mealtime insulin doses compared with those treated with glargine (Figure 4) [Rosenstock J et al. Diabetes Care 2013].
Glycemic Control After 8 Weeks of LY2605541 Versus Glargine in Type 1 Diabetes
Reproduced from Rosenstock J et al. Better Glycemic Control and Weight Loss With the Novel Long-Acting Basal Insulin LY2605541 Compared With Insulin Glargine in Type 1 Diabetes: A randomized, crossover study. Diabetes Care 2013;36:522–528. With permission from the American Diabetes Association.
The new bolus insulins offer faster onset of action and more rapid clearance. Faster onset allows immediate premeal dosing, better postprandial glycemic control, and better management of glucose levels in patients with unpredictable food intake. With more rapid clearance, there is less late postbolus hypoglycemia, reduced insulin stacking, and potentially less weight gain. When currently available rapid-acting insulins are used in conjunction with hyaluronidase there is no increased injection-site pain, erythema, or induration, and no other increased adverse effects. Hyaluronidase accelerates the absorption and action of coinjected regular human insulin and the rapid-acting insulin analog lispro. Lispro with hyaluronidase produces earlier and greater peak insulin concentrations, improves postprandial glycemic control [Hompesch M et al. Diabetes Care 2011], and provides for accelerated insulin exposure with a faster onset and shorter duration of insulin action [Morrow L et al. Diabetes Care 2013]. The new insulins look promising, but long-term studies are needed to confirm efficacy and safety.
Celeste P. Durnwald, MD, University of Pennsylvania, Philadelphia, Pennsylvania, USA, discussed guidelines and experience with the long-acting insulin analogs in pregnancy. The rapid-acting insulin analogs lispro, aspart, and glulisine offer fast action, less postprandial hypoglycemia, and less variability in absorption at the injection site relative to regular insulin
Lispro and aspart offer similar efficacy but not consistent glycemic benefit. In the compilation of published studies to date, there are no major maternal or fetal safety concerns, and both have been adapted into clinical use. Glargine and detemir are long-acting analogues that have the advantage of once-daily dosing, lengthy constant release of insulin, and decreased hypoglycemia, particularly at night. Data on glargine are limited, and this drug cannot be recommended in pregnancy. Treatment with insulin detemir resulted in lower fasting plasma glucose levels and produced similar HbA1C levels in pregnant women with type 1 diabetes compared with neutral protamine Hagedorn (NPH) insulin. Rates of hypoglycemia were comparable [Mathiesen ER et al. Diabetes Care 2012]. Insulin detemir could be considered on an individualized basis in those women with significant risk of hypoglycemia. However, NPH remains the basal insulin of choice in pregnancy. In all cases, it is important to balance tight glycemic control and maternal hypoglycemia, and then match physiologic needs and pharmacokinetics.
- © 2013 MD Conference Express®