Summary
Obesity-related glomerulopathy (ORG) is distinct from idiopathic focal segmental glomerulosclerosis, and is an emerging epidemic clinical entity [Kambham N et al. Kidney Int 2001]. This article discusses evidence for a link between obesity and the development and progression of chronic kidney disease (CKD) and end-stage renal disease, athological patterns of injury relevant to ORG, an overview of glycemic control in patients with diabetic kidney disease, as well as a relationship among hypertension, diabetes, and CKD .
- Obesity
- Diabetes & Kidney Disease
- Hypertensive Disease
- Hyperglycemia/Hypoglycemia Renal Disease
- Hypertension & Kidney Disease
- Diabetes Mellitus
- Obesity
- Diabetes & Kidney Disease
- Hypertensive Disease
- Hyperglycemia/Hypoglycemia
- Endocrinology
- Diabetes & Metabolic Syndrome
- Renal Disease
- Hypertension & Kidney Disease
- Diabetes Mellitus
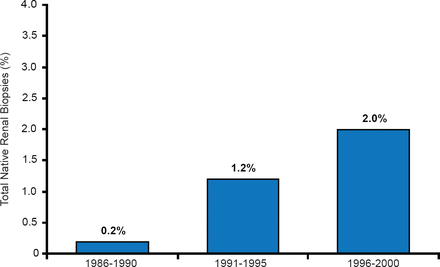
Obesity-related glomerulopathy (ORG) is distinct from idiopathic focal segmental glomerulosclerosis, and is an emerging epidemic clinical entity (Figure 1) [Kambham N et al. Kidney Int 2001].
Incidence of Obesity-Related Glomerulopathy Over Time
Adapted from Kambham N et al. Kidney Int 2001.
Allon Friedman, MD, Indiana University School of Medicine, Indianapolis, Indiana, USA, presented evidence for a link between obesity and the development and progression of chronic kidney disease (CKD) and end-stage renal disease (ESRD). Elevated glomerular filtration rate (GFR; so-called glomerular hyperfiltration) and albuminuria are commonly observed in obesity and are also known to predict loss of renal function in diabetes [de Jong PE et al. Int J Obesity 2002]. Renin angiotensin aldosterone system (RAAS) hormones are also elevated in obese individuals [Tuck ML et al. N Engl J Med 1981] and may contribute to glomerular and systemic hypertension and possibly kidney damage. Preliminary data suggest that the best treatments for obesity-related kidney disease involve weight loss (either surgical or medical) and inhibition of the RAAS axis. Dr. Friedman cautions, however, that the evidence for a causal link between obesity and CKD is limited at present due to the lack of rigorous experimental and interventional studies in obese individuals with CKD.
The prevalence of obesity-related kidney disease is not increasing at the same rate as the overweight and obese population in the United States, indicating that obesity alone may not be sufficient cause for CKD. Differences in glomerular density has been offered as an explanation for this disparity, with low glomerular density, in addition to marked glomerulomegaly, being potentially characteristic histologic findings in patients with obesity-related glomerulopathy (ORG) [Tsuboi N et al. Clin J Am Soc Nephrol 2012].
Charles E. Alpers, MD, University of Washington Medical Center, Seattle, Washington, USA, discussed the pathological patterns of injury relevant to ORG, which encompasses glomerulomegaly and focal and segmental glomerulosclerosis (FSGS). Glomerulomegaly is associated with increased GFR, renal hypertrophy, increased renal blood flow, and proteinuria. Such histological findings have also associated with congenital renal hypoplasia, hypertension, and diabetic nephropathy. Glomerulomegaly occurs more often in Australian Aboriginals and African Americans and often occurs as a compensatory mechanism in response to a reduction in renal mass. It is believed to primarily be the result of vascular dilatation and proliferation of the specialized cells (mesangial) that regulate the blood flow through the kidney capillaries.
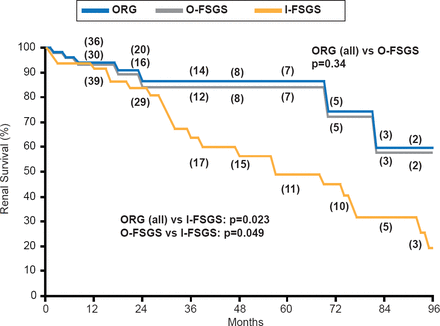
FSGS is associated with podocyte effacement. ORG, which in many cases can be considered a form of secondary FSGS, and primary FSGS have distinct morphologic features. The extent of segmental sclerosis and fusion of podocyte foot processes are significantly lower and glomerulomegaly significantly greater in ORG compared with FSGS. Renal survival is better in ORG. However, renal survival outcomes for obesity-related FSGS are significantly better than outcomes for idiopathic FSGS, which is characterized by higher levels of defined as massive proteinuria, higher prevalence of acute onset nephrotic syndrome, faster progression to ESRD, no glomerulomegaly, and more extensive 100% foot process effacement [Kambham N et al. Kidney Intl 2001].
George L. Bakris, MD, University of Chicago Medicine, Chicago, Illinois, USA, updated the audience concerning the relationship among hypertension, diabetes, and CKD.
CKD is an independent risk factor for mortality. The risk of a coronary event is 10-times higher in individuals with diabetes and CKD compared with individuals without either disease (Figure 2) [Tonelli M et al. Lancet 2012; Polonsky TS, Bakris GL. Lancet 2012]. CKD is also associated with increased risk of ESRD in individuals with and without diabetes [Fox C et al. Lancet 2012].
Risk of Coronary Events in Patients With Chronic Kidney Disease Versus Diabetes
I-FSGS=idiopathic focal and segmental glomerulosclerosis; O-FSGS=obesity-related focal and segmental glomerulosclerosis; ORG=obesity-related glomerulopathy.
Reproduced from Tonelli M et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012;380(9844):807–814. With permission from Elsevier.
It has been reported that microalbuminuria in the presence of hypertension indicates established abnormalities of glomerular structure in patients with type 1 diabetes (T1D) and may be a good marker for nephropathy in patients with diabetes mellitus [Chavers BM et al. N Engl J Med 1989]. Dr. Bakris feels that microalbuminuria reduction should not be accepted as surrogate for slowing the rate of nephropathy progression in the presence of reduced blood pressure, since 64% of the patients that develop microalbuminuria revert to normoalbuminuria [Steinke JM et al. Diabetes 2005].
Nephropathy and retinopathy remain important complications of T1D and early blockade of the RAAS has been suggested as way to slow the progression. However, in a recent clinical trial [Renin Angiotensin System Study; NCT00143949] the early use of RAAS inhibitors in patients with T1D did not slow nephropathy progression, although it did slow the progression of retinopathy [Mauer M et al. N Engl J Med 2009]. However, in another study, RAAS inhibition slowed diabetic nephropathy in patients with advanced proteinuric disease (>300 mg/24 hours) [Kunz R et al. Ann Intern Med 2008].
With respect to hypertension, blood pressure goals for patients with diabetes have been reduced to <140/90 or 80 mm Hg based on the outcomes from the ACCORD [ACCORD Study Group. N Engl J Med 2011], INVEST [Cooper-DeHoff RM et al. JAMA 2010], and other trials, and these changes have been adopted in the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Guidelines [Wheeler D, Becker GJ. Kidney Int 2012].
Approximately 40% of persons with diabetes develop diabetic kidney disease (DKD), which presents as albuminuria, impaired GFR, or both, and the prevalence is increasing [de Boer IH et al. JAMA 2011]. Robert G. Nelson, MD, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, Arizona, USA, presented an overview of glycemic control in these patients.
The long-term risk of an impaired GFR is significantly lower among persons treated early in the course of T1D with intensive therapy versus those treated with conventional therapy [DCCT/EDIC Research Group. N Engl J Med 2011]. However, there is a lack of randomized controlled trial evidence for intensive therapy for persons with DKD, as they are often excluded from these studies. In the Diabetes Control and Complications Trial [DCCT], intensive glycemic control increased the risk of severe hypoglycemia in the short term, and for this reason its benefits must be balanced against the potential harm. Clinical practice guidelines offered by the American Diabetes Association state that less stringent HbA1C goals (such as <8%) may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced vascular complications, and extensive comorbid conditions.
- © 2013 MD Conference Express®