Summary
Three-year data from the Efficacy of Exenatide Once Weekly and Once-Daily Insulin Glargine in Patients With Metformin Alone or in Combination With Sulfonylurea trial [DURATION-3; NCT00641056] have confirmed previously reported 26- and 84-week results demonstrating the superiority of the exenatide regimen for glycemic and weight control, and lowered risk of hypoglycemia [Diamant M et al. Lancet 2010; Diamant M et al. Diabetes Care 2012].
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia Diabetes & Endocrinology Clinical Trials
- Insulin
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes & Endocrinology Clinical Trials
- Insulin
Three-year data from the Efficacy of Exenatide Once Weekly and Once-Daily Insulin Glargine in Patients With Metformin Alone or in Combination With Sulfonylurea trial [DURATION-3; NCT00641056] have confirmed previously reported 26- and 84-week results demonstrating the superiority of the exenatide regimen for glycemic and weight control, and lowered risk of hypoglycemia [Diamant M et al. Lancet 2010; Diamant M et al. Diabetes Care 2012].
DURATION-3 was an open-label, randomized, controlled study of patients with type 2 diabetes mellitus comparing once-weekly injection of exenatide, a glucagon-like peptide-1 receptor agonist, to titrated insulin glargine. Michael Trautmann, MD, Diabetologist and Consultant, Hamburg, Germany, reported the 3-year results, noting that this study was unique in that it compared the two injectable therapies over 3 years in patients who had not achieved an HbA1C level of <7% during treatment with metformin alone or in combination with sulfonylurea.
The 456 enrolled patients were randomized to exenatide 2 mg QW (n=233) or titrated insulin glargine QD (n=223). All patients received metformin with or without sulfonylurea. The study consisted of a 26-week core study period followed by a 130-week controlled extension period. A substantial proportion of participants completed the 156-week regimen (60% in the exenatide arm and 66% in the insulin glargine arm). The baseline characteristics of the intention-to-treat (ITT) subjects and the completers were similar, notably concerning HbA1C level (∼8.3%) and duration of diabetes (∼8 years).
Dr. Trautmann reported that in the ITT population, mean HbA1C levels at 3 years were significantly lower with exenatide (7.3±0.07%) versus insulin glargine (7.5±0.07%; p=0.033; Figure 1). Similarly, in the completer population, mean HbA1C levels at 3 years were significantly lower with exenatide (7.1±0.08%) versus insulin glargine (7.4±0.08%; p=0.022). The similarity of the findings in the ITT and completer populations emphasizes the representative nature of the 3-year data. Furthermore, significantly more patients in the exenatide arm achieved HbA1C targets of ≤6.5% at 3 years (24% vs 15%; p=0.02 [ITT population]; 28% vs 18% [completer population]).
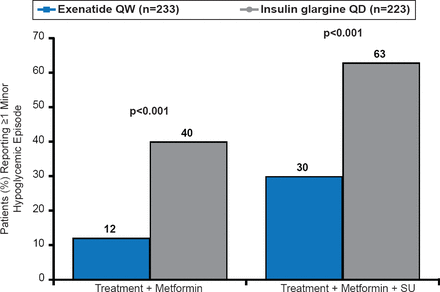
Incidence of Minor Hypoglycemia at 3 Years
QD=once daily; QW=once weekly; SU=sulfonylurea.
Reproduced with permission from M Trautman, MD.
Patients in the exenatide group gained body weight (mean, −2.49±0.28 kg) while those receiving insulin glargine lost body weight (mean, +2.01±0.28 kg). There was a significant difference between the groups for the change in body weight from baseline to 3 years (mean difference, −4.51±0.37 kg; p<0.001).
Sixty-eight percent of patients in the exenatide arm displayed both reduced HbA1C and body weight at 3 years compared with only 34% in the insulin glargine arm. Fasting serum glucose was also significantly decreased in patients receiving exenatide (mean, −31.16 mg/dL) versus those receiving insulin glargine (mean, −47.74 mg/dL; p<0.001).
The safety profile of both drugs at 3 years echoed the previous 26- and 84-week results. While subjects receiving exenatide were more prone to gastrointestinal maladies including nausea, vomiting, and diarrhea than those receiving insulin glargine (16% vs 2%; 6% vs 3%; 14% vs 7%, respectively), most adverse events occurred in the first 26 weeks in the exenatide group. Consistent with the better longer-term tolerance of exenatide, the positive rate for anti-exenatide antibodies decreased from 56% at 26 weeks to 19% at 3 years.
Exenatide was associated with significantly fewer incidents of hypoglycemia than insulin glargine when used in combination with metformin alone (p<0.001) or with metformin plus sulfonylurea (p<0.001; Figure 1).
Dr. Trautmann concluded that exenatide is superior to insulin glargine in terms of sustained glycemic control and weight loss, and in lessening the risk of hypoglycemia.
- © 2013 MD Conference Express®