Summary
Linagliptin is an oral hypoglycemic compound used in the treatment of T2DM. The target of the drug is dipeptidyl peptidase 4, an enzyme that is important in various functions including the metabolism of glucose. This article discusses the cardiovascular (CV) safety of linagliptin in nearly 9500 patients with type 2 diabetes mellitus with a wide spectrum of CV risk and treatment history, based on an analysis of 19 randomized controlled trials.
- Cerebrovascular Disease
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Myocardial Infarction Cerebrovascular Disease
- Diabetes & Endocrinology Clinical Trials
- Cerebrovascular Disease
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Myocardial Infarction
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes & Endocrinology Clinical Trials
Odd Eric Johansen, MD, Global Senior Medical Director, Metabolism (Diabetes), Boehringer Ingelheim, Frankfurt, Germany, reported on the cardiovascular (CV) safety of linagliptin in nearly 9500 patients with type 2 diabetes mellitus (T2DM) with a wide spectrum of CV risk and treatment history, based on an analysis of 19 randomized controlled trials.
Linagliptin is an oral hypoglycemic compound used in the treatment of T2DM. The target of the drug is dipeptidyl peptidase 4, an enzyme that is important in various functions including the metabolism of glucose. Previous studies have provided evidence that linagliptin is not associated with an increased risk of CV events [Graefe-Mody EU et al. Curr Med Res Opin 2009; Deacon CF, Holst JJ. Expert Opin Investig Drugs 2010; Friedrich C et al. Eur J Drug Metab Pharmacokinet 2011; Johansen OE et al. Cardiovasc Diabetol 2012].
Diabetes almost doubles the risk of a number of vascular diseases including coronary artery disease, myocardial infarction (MI), ischemic stroke, and hemorrhagic stroke. The risk of stroke for individuals with diabetes is similar to those with a history of coronary heart disease [Grundy SM et al. Circulation 2004].
Dr. Johansen reported on the comparison of the incidence of CV events and CV mortality in 9459 T2DM patients who had been treated with linagliptin (n=5847; 5687 treated with 5 mg, 160 treated with 10 mg) with a comparator group of patients who did not receive the drug (n=3612; placebo 2675, glimepiride 775, voglibose 162). The data were derived from 19 double-blind randomized controlled trials that each lasted a minimum of 12 weeks. Adverse CV events were identified using the Standard Medical Dictionary for Regulatory Activities and the identified trigger events and accompanying data were prospectively adjudicated by a blinded independent expert committee.
The primary endpoint was a composite of CV death, nonfatal stroke, nonfatal MI, and hospitalization for unstable angina pectoris. Secondary CV endpoints included CV death, fatal stroke, and fatal MI. The cumulative exposure (person-years) was 4421.3 for patients receiving linagliptin and 3254.7 for the comparator patients.
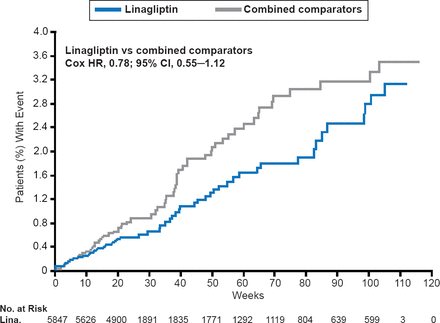
The frequency of primary outcome events was similar in the linagliptin-treated patients (n=60; 1.0%) and the comparator group (n=62; placebo 36, active comparators 26; 1.7%). Incidence rates of the primary endpoint per 1000 years at risk were lower for patients treated with linagliptin (13.4) than for the comparator patients (18.9), as was the hazard ratio (0.78; 95% CI, 0.55 to 1.12; Figure 1 and Table 1). Tertiary endpoints were similar in both groups.
Drug Exposure and Incident/Incidence Rates of CV Endpoints and Mortality
Primary Composite Endpoint of CV Death, Nonfatal MI, Stroke, and HUA Over Time
Reproduced with permission from OE Johansen, MD.
Dr. Johansen and colleagues concluded that the pooled data obtained from a large number of T2DM patients with a spectrum of CV risk and a varied treatment history ranging from none to insulin dependence bolsters the view that linagliptin does not increase the risk of adverse CV events. This retrospective assurance now paves the way for prospective studies [CAROLINA, NCT01243424 and CARMELINA, NCT01897532] designed to assess the therapeutic benefit of linagliptin in T2DM.
- © 2013 MD Conference Express®