Summary
This article provides an overview of the fundamentals of recent guidelines concerning sentinel lymph node biopsy (SLNB) in melanoma. It goes on to discuss the risk of recurrence associated with positive, and negative SLNB results and the value of SLNB for thin melanomas (T1, Breslow thickness =1.00 mm),
- Oncology Guidelines
- Soft Tissue Cancers
- Lymphatic Diseases
- Oncology Guidelines
- Soft Tissue Cancers
- Lymphatic Diseases
- Oncology
GUIDELINE FUNDAMENTALS FOR SLNB IN MELANOMA
Vernon K. Sondak, MD, Chair, Moffitt Cancer Center, Tampa, Florida, USA, provided an overview of the fundamentals of recent guidelines concerning sentinel lymph node biopsy (SLNB) in melanoma. Lymphatic flow drains from skin regions to one or a few SLNs. If the SLNs are negative for melanoma, it is unlikely that other nodes will contain cancer cells.
Detailed examination of biopsied SLN tissue is possible; serial sectioning and immunohistochemistry can reveal even a handful of tumor cells within the lymph node, which can be indicative of stage III melanoma. Identification can be followed by surgical excision utilizing a radioactive tracer and a dye to precisely target the region of concern.
There are several potential benefits of SLNB:
-
▪ Staging is paramount; accurate tumor staging aids the prognosis of the risk of recurrence and melanoma-related death
-
▪ Prolonged relapse-free survival
-
▪ Lymphadenectomy for micrometastatic disease, rather than waiting for classical palpation/radiologic clinical detection of metastasis, can reduce regional failure
-
▪ Lymphadenectomy for micrometastatic disease is associated with fewer complications than surgery for clinically evident disease, and less lymphedema
The ultimate goal of SLNB is improved melanoma-specific survival. Lymphadenectomy for micrometastatic disease and early use of adjuvant therapy, rather than treatment upon clinical detection of the tumor, could increase the cure rate.
However, SLNB poses potential risks. Seroma and wound infection may require drainage or antibiotic therapy. A false-negative result can delay recognition, and hence treatment, of regional lymph node metastasis. In-transit recurrence caused by interruption of the lymphatic pathway was once thought capable of trapping tumor cells between the primary location and the lymphatic basin, although this may not be an actual concern. However, lymphedema can result from interrupted flow of lymphatic drainage. Finally, nerve injury can occur, particularly in the head and neck region, since lymph nodes often transit alongside nerves. The potential risks can increase mortality of completion lymphadenectomy (a complete lymph node dissection following a positive SLNB) in contrast to a therapeutic lymphadenectomy following palpable detection of tumor.
Recognition of the benefits and risks of SLNB, particularly for intermediate thickness melanoma, has prompted the incorporation of relevant guidelines in the national clinical practice guidelines of many countries. In July 2012, the American Society of Clinical Oncology (ASCO) and the Society of Surgical Oncology (SSO) issued evidence-based joint clinical practice guidelines on SLNB for melanoma (Table 1) [Wong SL et al. Annals Surg Oncol 2012; J Clin Oncol 2012].
Summary of ASCO/SSO Joint Guidelines for SLNB
RISK OF RECURRENCE ASSOCIATED WITH POSITIVE AND NEGATIVE SLNB RESULTS
Sandra L. Wong, MD, MS, University of Michigan, Ann Arbor, Michigan, USA, discussed the risk of recurrence associated with positive and negative SLNB results and their implications for adjuvant clinical trial design and enrollment.
The ASCO/SSO 2012 guidelines were largely based on a meta-analysis of the pertinent literature comprising 71 studies and 25,240 patients [Valsecchi ME et al. J Clin Oncol 2011] that focused on the indications for SLNB and the role of completion lymph node dissection (CLND). The overall false-negative rate was 12.5% and the post-test probability negative (PTPN) rate was 3.4%. The PTPN, which differs from a false-negative rate, is calculated as the number of patients with negative SLNB who recurred divided by all patients with negative SLNB. Both statistics are important in defining recurrence, particularly the risk that a negative result was incorrect.
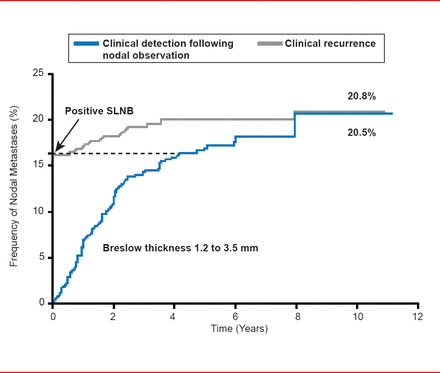
However, the PTPN result may be the more important of the two values in assessing the risk of recurrence after a negative SLNB result. Data from studies examining the cumulative incidence of detection of nodal metastases detection by SLNB versus observation have demonstrated a similar rate (∼21%) after 10 years, indicating the accuracy of the biopsy approach as a predictor of the development of nodal metastases (Figure 1). The appreciable time gap between biopsy-mediated and clinical detection supports the prognostic value of CLND, which is currently recommended for SLNB-positive patients.
However, ∼8% of patients who have CLND still experience disease recurrence, indicating that CLND does not provide an absolute guarantee that recurrence will not occur. This has prompted discussion of the therapeutic value of CLND. This issue may be resolved following completion of the ongoing Multicenter Selective Lymphadenectomy Trial-II [MSLT-II; NCT00297895] a randomized, open-label study, which will compare CLND with observation in patients with positive SLNB. But the current evidence suggests that patients with positive SLNB undergo CLND, or discuss options such as a clinical trial to evaluate alternative therapies.
Cumulative Incidence of Nodal Metastases: SLNB Versus Observation
Reproduced with permission from SL Wong, MD, MS.
SLNB results have predictive and prognostic value, but not direct therapeutic value in terms of a survival benefit. Importantly, prediction allows patient stratification, which is crucial in clinical trial design and enrollment.
SLNB FOR THIN MELANOMA
Jeffrey E. Gershenwald, MD, University of Texas MD Anderson Cancer Center, Houston, Texas, USA, discussed the value of SLNB for thin melanomas (T1, Breslow thickness ≤1.00 mm). The current American Joint Committee on Cancer classification defines thin melanoma as the absence of ulceration and mitotic activity throughout the tumor <1 mitosis/mm2 (T1a) or in the presence of ulceration or >1 mitosis/mm2 throughout the tumor (T1b) [Balch CM et al. J Clin Oncol 2009].
Mitotic rate increases fairly linearly and steeply in tumors up to ∼3 mm in thickness and then slows with increasing tumor thickness. Patients can have a wide range of mitotic activity in thin tumors [Thompson JF et al. J Clin Oncol 2011].
Up to 70% of newly diagnosed melanomas are thin (ie, ≤1 mm tumor thickness). Most have a generally excellent prognosis, with overall survival at 10 years of 92% [Balch CM et al. J Clin Oncol 2009;]. However, some patients develop clinically evident regional metastasis, usually after a long time [Thompson JF, Shaw HM. Ann Surg Oncol 2006]. The high incidence despite overall low risk translates into a significant absolute number of potential individuals affected, and so is an important public health issue [Andtbacka R et al. J Natl Compr Cancer Network 2009].
SLNB is the standard of care for patients with intermediate thickness melanoma and is recommended for nearly all patients with melanomas ≥1 mm thick. However, use of SLNB in patients with thin melanoma is controversial due to the overall low risk of nodal metastasis, uncertain prognostic value of a positive SLNB, and associated risks and costs. There is insufficient evidence supporting routine SLNB for thin melanomas (T1, <1 mm Breslow thickness), although the approach may be considered in select cases with high-risk features, when the benefits of pathologic staging outweigh procedural risks. However, it is noteworthy that in formulating the ASCO/SSO guidelines, studies that explored risk of positive SLNB but that did not have follow-up data were excluded, which limited the data available. Long-term follow-up is important to assess the prognostic impact of regional nodal staging [Gershenwald JE et al. Ann Surg Oncol 2012; Wong SL et al. Annals Surg Oncol 2012; Wong SL et al. J Clin Oncol 2012]. Some patients have an incidence of positive SLNB that is sufficiently high enough to perhaps justify the procedure. In a pooled series, the overall probability of positive SLNB in patients with melanoma <1 mm who underwent SLNB was ∼5% (2% to 4% for <0.76 mm and 6% to 11% for 0.76 to 0.99 mm) [Andtbacka R et al. J Natl Compr Cancer Network 2009; Gershenwald JF et al. Ann Surg Oncol 2012].
The available data highlight the need to determine the appropriate threshold thickness of melanoma when deciding on SLNB. No consensus currently exists. Clinicians should discuss the concept of SLNB with all thin melanoma patients, including providing an explanation of why SLNB is not recommended. SLNB likely provides important prognostic information in a subset of thin melanoma patients with melanomas 0.76 to 0.99 mm in thickness, but is not recommended for the overwhelming majority of patients with melanomas <76 mm in thickness [Gershenwald JF et al. Ann Surg Oncol 2012].
- © 2013 MD Conference Express®