Summary
One of the most important advances in the treatment of acute lymphoblastic leukemia (ALL) is the development of monoclonal antibody therapies. Multiple derivatives of this therapy have been developed, each with unique targets and mechanisms of action. This article discusses the monoclonal antibody-based therapies for the treatment of ALL, ALL in older adolescent and young adult patients, and minimal residual disease in ALL.
- Leukemia
- Oncology
- Leukemia
One of the most important advances in the treatment of acute lymphoblastic leukemia (ALL) is the development of monoclonal antibody therapies. Multiple derivatives of this therapy have been developed, each with unique targets and mechanisms of action. Mark R. Litzow, MD, Mayo Clinic, Rochester, Minnesota, USA, presented on monoclonal antibody-based therapies for the treatment of ALL.
The humanized CD22 immunoglobulin G1 antibody, epratuzumab, has demonstrated efficacy as monotherapy and in combination with rituximab with or without chemotherapy in non-Hodgkin lymphoma. The first trial that evaluated the use of epratuzumab in ALL was performed in children and used weekly epratuzumab in combination with chemotherapy [Raetz E et al. J Clin Oncol 2008]. In this study, treatment with epratuzumab resulted in a complete response (CR) in 9 of 15 patients. In the Phase 2 S0910 trial [NCT00945815], 35 patients received epratuzumab in combination with clofarabine and cytarabine; CR rate was 45%.
Dr. Litzow discussed the mechanism of action of antibody-drug conjugates and highlighted a study evaluating inotuzumab ozogamicin (IO), which is an anti-CD22 antibody conjugated to calicheamicin [NCT01363297; O'Brien S et al. ASH 2012 (abstr 671)]. In this study, patients with relapsed or refractory ALL received either monthly IO (1.8 mg/m2) or a weekly regimen (0.8 mg/m2 followed by 0.5 mg/m2). The overall response rate was similar among both arms at 57% and 58%. Adverse events included drug fever, hypotension, and hepatic toxicity as measured by increased bilirubin and liver enzymes, which occurred more frequently with monthly dosing of IO. In this trial, 22 out of 49 patients in the monthly IO arm and 11 out of 40 patients in the weekly IO arm were able to undergo allogeneic stem cell transplant (ASCT).
Dr. Litzow presented data from a Phase 1 trial evaluating moxetumomab pasudotox, a novel anti-CD22 immunotoxin, in pediatric patients with ALL [NCT00659425; Wayne AS et al. Blood 2011 (abstr 248)]. In this trial, 21 heavily pretreated patients received moxetumomab pasudotox 6 times Q3W as monotherapy or in combination with dexamethasone, to decrease the risk of capillary leak syndrome. The response rate was 30% and adverse events included cytopenias, fever, elevated transaminase levels, tachycardia, and capillary leak syndrome.
Bispecific T-cell engaging (BiTE) antibodies are novel agents that guide cytotoxic polyclonal T cells to engage cancer cells that express CD19 by binding to cell surface receptors on both cells, bringing them in close proximity. The BiTE antibody blinatumomab was evaluated in adult patients with relapsed or refractory ALL [NCT01209286; Topp MS et al. Blood 2012 (abstr 670)]. Cytokine release syndrome and central nervous system events such as encephalopathy and seizures are important reversible toxicities that require treatment interruption or discontinuation. Hematologic response was observed within 2 cycles of treatment, with a CR rate of 69%. Based on these promising results, Dr. Litzow introduced the upcoming E1910 Phase 3 trial, which will evaluate blinatumomab in newly diagnosed patients with ALL.
Dr. Litzow concluded with a discussion on chimeric antigen receptor-modified T cells (CART cells) in which T cells are collected from the patient, transduced by a retrovirus with CAR genes, and expanded in the laboratory, then infused back into the patient [Lipowska-Bhalla G et al. Cancer Immuno Immunother 2012]. In the Pedi CART-19 study [Grupp SA et al. N Engl J Med 2013], two pediatric patients with relapsed or refractory ALL were treated with CART cells. The CART cells were observed in the cerebrospinal fluid for at least 6 months. Dr. Litzow highlighted the cytokine “storm” that was observed in both patients, where multiple cytokines were elevated following the infusion of the CART cells. The CART study has now treated five heavily pretreated pediatric patients with refractory or relapsed ALL [NCT01626495; Rheingold S, Grupp S. Unpublished data]. At this time a total of four CRs and three complete cytogenic responses, up to 1 year out, have been achieved.
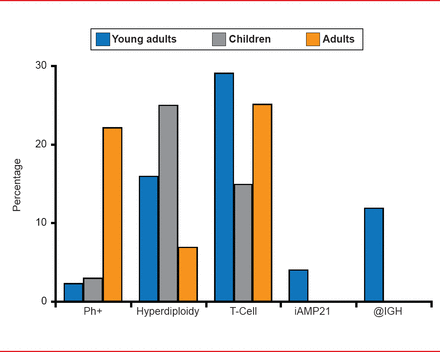
Anjali S. Advani, MD, Cleveland Clinic, Cleveland, Ohio, USA, discussed ALL in older adolescent and young adult patients (AYA). Although ALL is typically thought of as a pediatric disease, Dr. Advani pointed out that ∼25% of ALL patients fall between 16 and 39 years [Henderson ES et al. Leukemia, 7th Ed. WB Saunders Co. 2002]. Interestingly, the AYA patient population has unique cytogenetic and immunophenotypic features (Figure 1). In addition, it appears that the AYA population benefits more from pediatric-inspired regimens compared with conventional adult regimens. A recent study suggested that this effect may be due to the greater doses of steroids, vincristine, asparaginase, and intrathecal methotrexate in the pediatric-inspired regimens [Ram R et al. Am J Hematol 2012].
Unique Cytogenetic and Immunophenotypic Features of Adolescent/Young Adult ALL
ALL=acute lymphoblastic leukemia.
Dr. Advani raised the question of the age to which a pediatric regimen should be extended. The GRAAL2 study [Huguet F et al. J Clin Oncol 2009] evaluated the efficacy of a modified pediatric treatment regimen in 255 patients aged 15 to 60 years (median age 31 years). As compared with the adult LALA-94 trial, patients in GRAAL2 experienced an improved event-free survival and overall survival at Month 42. However, in the subgroup of patients aged ≥45 years in the GRAAL2 study, the overall survival benefit of a modified pediatric regimen appeared to decrease.
Dieter Hoelzer, MD, Johann Wolfgang Goethe University, Frankfurt, Germany, discussed minimal residual disease (MRD) in ALL; detection of leukemic blast cells not assessable by a microscopic bone marrow evaluation. MRD can be measured by flow cytometry and real-time quantitative polymerase chain reaction, depending on the target being assessed. Next generation sequencing might be a future method to detect MRD with a higher sensitivity.
In the treatment of patients with MRD, Prof. Hoelzer suggested that it is important to consider pretherapeutic factors and laboratory features at diagnosis, but also factors of MRD. For example, prognostic factors such as a high white blood cell count, disease subtype, timing of complete remission achievement, and cytogenetic and molecular aberrations can identify patients with different risks. In the GMALL study, patients that are standard risk with no high-risk factors comprise ∼50%, those that are high risk with one or more risk factors are ∼33%, and those patients that are very high risk with the BCR-ABL fusion protein represent ∼17% [personal communication]. High-risk and very high-risk patients should most likely receive a stem cell transplant (SCT) following the first complete remission. The next step would be to measure MRD and stratify patients based on their MRD status. If patients are standard risk and are MRD-negative, then chemotherapy should be administered. If patients are standard risk or high or very high risk and MRD-positive, then SCT should be initiated.
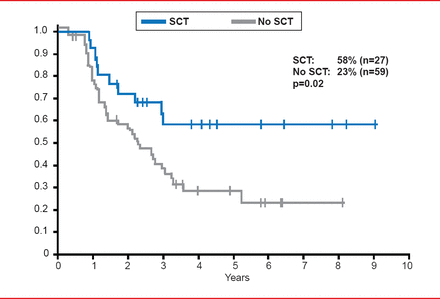
Prof. Hoelzer highlighted that MRD-positive patients that undergo SCT have improved survival outcomes compared with patients that do not receive SCT. In the GMALL study of standard risk, MRD-positive ALL patients, patients that received SCT had an overall survival rate of 58%, as compared with 23% in patients that did not undergo SCT (p=0.02; Figure 2) [Gökbuget N et al. Blood 2012].
Survival Outcomes in MRD-Positive ALL Patients With or Without SCT
Gökbuget N et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012;120:1868–1876. With permission from the American Society of Hematology.
ALL research has led to the development of multiple novel agents that target different aspects of the disease. Currently, many of these agents have demonstrated promising results in early-phase clinical trials.
- © 2013 MD Conference Express®