Summary
An improved understanding of the biology of multiple myeloma (MM) and the identification of new drugs have improved the therapeutic approach. Myeloma consists of at least seven subtypes based on cytogenetics and molecular features. This article discusses the use of autologous stem cell transplant as a therapy for MM, approaches to patients with ‘double-refractory’ myeloma, and advances in diagnostic assays and monitoring of MM.
- Soft Tissue Cancers
- Multiple Myeloma
- Soft Tissue Cancers
- Oncology
- Multiple Myeloma
An improved understanding of the biology of multiple myeloma (MM) and the identification of new drugs have improved the therapeutic approach.
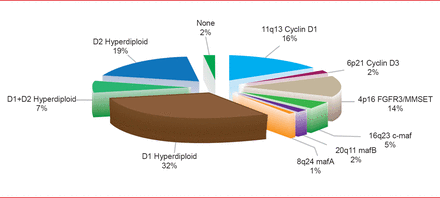
Myeloma consists of at least seven subtypes based on cytogenetics and molecular features, said Donne E. Reece, MD, Princess Margaret Cancer Center, Toronto, Ontario, Canada. The highest risk subtypes by fluorescence in situ hybridization (FISH) are t(4;14), t(14;16), deletion 17p identifies, and chromosome 1 abnormalities; all are recognized as adverse prognostic factors (Figure 1) [Chesi M, Bergsagel PL. Int J Hematol 2013].
Distribution of Genetic Subtypes of Untreated MM Using the TC Classification
Adapted from Chesi M, Bergsagel PL. Int J Hematol 2013.
An evolving treatment algorithm in the United States recommends treating younger individuals, particularly those with standard-risk disease, with regimens based on novel agents for variable periods of time; autologous stem cell transplant (ASCT) is considered optional based on patient preference and other factors. Some of these approaches advocate ASCT at the time of relapse.
The median progression-free survival (PFS) after ASCT has increased from 2 years with vincristine, doxorubicin, and dexamethasone (DEX) or thalidomide (THAL) plus DEX induction to 3 years when bortezomib (BORT) is used. BORT-containing regimens as induction also yield better response rates and overall survival (OS) compared with these older regimens. The inclusion of BORT, particularly in a 3-drug regimen, seems important for high-risk disease, as indicated by the recent integrated analysis of the four Phase 3 studies of BORT induction [Cavo M et al. Blood 2012 (abstr 749)].
Both maintenance and consolidation therapy further improve response rates and PFS but their impact on survival is less clear.
A meta-analysis of seven Phase 3 clinical trials of THAL as post-ASCT maintenance therapy concluded that THAL as a single agent or in conjunction with corticosteroids improves both PFS and OS [Nooka AK et al. Blood 2011 (abstr 1855)]. Lenalidomide (LEN) maintenance has been assessed in two Phase 3 trials, demonstrating significant prolongation of PFS and time to progression [Attal M et al. N Engl J Med 2012; McCarthy PL et al. N Engl J Med 2012].
Advantages of consolidation (moderately intensive combination therapy given for several cycles after recovery from ASCT) compared with long-term maintenance therapy include a finite period of treatment and, potentially, a lower and more predictable cost.
In the future, consolidation with a 3-drug combination would ideally be integrated into therapy, particularly in the high-risk setting.
In elderly patients, the addition of a novel agent (ie, THAL, BORT, or LEN) to melphalan and prednisone results in a better antimyeloma effect, although the incidence of Grade 3/4 toxicity is relatively high. LEN plus weekly DEX is also a promising regimen in elderly patients.
APPROACHES TO PATIENTS WITH ‘DOUBLE-REFRACTORY’ MYELOMA
Patients with MM that is refractory to LEN and BORT have a poor prognosis, and new therapeutic strategies are needed in this challenging population, said Robert Z. Orlowski, PhD, MD, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Potent analogs of existing MM drugs, such as carfilzomib and pomalidomide (POM), have demonstrated clinical efficacy in the double-refractory setting, resulting in recent regulatory approval of both drugs. In a multicenter single-arm Phase 2 trial of single-agent carfilzomib in 257 patients with relapsed or refractory MM, the overall response rate (ORR) was 15.4% in those with double-refractory disease, with a median duration of response of 7.8 months [Siegel DS et al. Blood 2012]. Median OS was 15.6 months in the overall population and 11.9 months in the double-refractory subgroup.
POM has been shown to be active against double-refractory MM in several Phase 2 and 3 trials. The Intergroup Francophone du Myélome randomly assigned 84 patients with relapsed/refractory MM to receive POM with weekly DEX [Leleu X et al. Blood 2013]. In the 64 patients with double-refractory disease, ORR was 31%, PFS was 3.8 months, and OS was 13.8 months.
In the MM-003 Phase 3 trial, patients were randomly assigned 2:1 to receive POM and low-dose DEX or single-agent high-dose DEX [Dimopoulos MA et al. Blood 2012 (abstr LBA-6)]. In 329 patients with double-refractory disease, median PFS in the POM arm was 3.2 versus 1.7 months in the high-dose DEX arm (p<0.001), and median OS was not reached in the POM arm whereas median OS in the high-dose DEX arm was 7.4 months (p=0.003).
A novel strategy may be to target kinesin-spindle protein (KSP). ARRY-520 is a potent, highly selective KSP inhibitor; a Phase 2 study was conducted by using ARRY-520 both as a single agent (Cohort 1) and in combination with low-dose DEX (Cohort 2) [Shah JJ et al. Blood 2012 (abstr 449)]. After a median treatment time of 3.9 months, ORR was 22% and the median duration of response was 5.4 months. The most frequent Grade 3 or 4 adverse events included neutropenia and thrombocytopenia. In Cohort 1, 53% had disease refractory to BORT and 75% had disease refractory to LEN [Lonial S et al. IMW 2013 (poster P-224)]. Of 32 Cohort 1 patients with assessable response, ORR was 19% with five partial responses (16%) [Shah JJ et al. Blood 2012 (abstr 449)].
Daratumumab is an investigational human monoclonal antibody that has received Breakthrough Therapy designation from the United States Food and Drug Administration for the treatment of patients with MM who have received at least three prior lines of therapy or who are double refractory to a proteasome inhibitor and an immunomodulatory agent. A Phase 1/2 dose-escalation study of 32 patients with relapsed MM showed at least a minimal response in patients who received 4 mg/kg of daratumumab or higher, with no major safety issues [Plesner T et al. Blood 2012 (abstr 73)].
ADVANCES IN DIAGNOSTIC ASSAYS AND MONITORING OF MM
Jesús F. San-Miguel, MD, PhD, University of Salamanca, Salamanca, Spain, discussed new tools in the diagnosis and monitoring of MM. Conventional morphology, protein electrophoresis, and skeletal survey are standard techniques, but novel cellular, serologic, and imaging assays have found their way into the clinic.
A patient with suspected MM should undergo a unilateral bone marrow aspirate and/or biopsy, with detection of ≥10% of plasma cells confirmatory of diagnosis. Serum-free light chain and new heavy/light chain assays are valuable for diagnosis and follow-up of oligosecretory myelomas; although not a substitute for the 24-hour urine assay.
Fluorescence in situ hybridization analysis on purified plasma cells is mandatory at baseline for risk stratification; it should only be repeated at relapse/progression for those patients classified as genetic standard risk at diagnosis.
Magnetic resonance imaging (MRI) is mandatory in four instances: for a presumed diagnosis of solitary plasmocytoma, detailed evaluation of a painful skeletal area, suspicion of cord compression, and before kyphoplasty. MRI may become a standard in the future for identification of occult bone disease in smoldering myeloma, as it can predict for faster progression to symptomatic disease. A computed tomography (CT) scan should be considered when MRI is unavailable or contraindicated. CT is also valuable to clarify lytic lesion in the ribs, sternum, and scapulae, as well as to assist planning of radiotherapy or surgery. Low-dose whole body CT may become a new standard for bone disease assessment. PET-scan is particulary valuable for evaluation of extramedulary involvement.
Flow cytometry immunophenotyping and real-time quantitative polymerase chain reaction have contributed to the evaluation of minimal residual disease, helping to define high-quality responses (immunophenotypic and molecular remission) that are associated with longer survival, and may also be used to monitor treatment efficacy. Next generation sequencing may represent a valuable alternative for molecular evaluation of minimal residual disease.
- © 2013 MD Conference Express®