Summary
There was no significant difference in 3-year disease-free survival in sequential treatment of paclitaxel and the oral fluoropyrimidine tegafur-uracil or paclitaxel and another fluoropyrimidine, S-1, in serosa-invading gastric cancer. This article presents data from the Adjuvant Paclitaxel Followed by Oral Fluorinated Pyrimidines for Locally Advanced Gastric Cancer trial [SAMIT; Yoshida K et al. J Clin Oncol 2013 (suppl; abstr LBA4002)].
- Oncology Clinical Trials
- Gastrointestinal Cancers
- Oncology Clinical Trials
- Gastrointestinal Cancers
- Oncology
There was no significant difference in 3-year disease-free survival (DFS) in sequential treatment of paclitaxel (PAC) and the oral fluoropyrimidine tegafur-uracil (UFT) or paclitaxel and another fluoropyrimidine, S-1, in serosa-invading gastric cancer. Kazuhiro Yoshida, MD, PhD, Gifu University School of Medicine, Gifu, Japan, presented data from the Adjuvant Paclitaxel Followed by Oral Fluorinated Pyrimidines for Locally Advanced Gastric Cancer trial [SAMIT; Yoshida K et al. J Clin Oncol 2013 (suppl; abstr LBA4002)].
In Japan, the standard adjuvant chemotherapy for the treatment of gastric cancer was UFT [Oba K et al. J Chemother 2006], until results of the ACTS-GC trial resulted in the replacement of UFT by S-1 [Sakuramoto S et al. N Engl J Med 2007]. However, Prof. Yoshida pointed out that UFT and S-1 have not been evaluated head-to-head in a clinical trial. In addition, PAC has demonstrated efficacy in advanced gastric cancer. The hypothesis of the SAMIT trial was to demonstrate the superiority of PAC followed by UFT or S-1 compared with no PAC use and the noninferiority of UFT to S-1.
In the multicenter, Phase 3 trial, 1495 patients with serosa-invading gastric cancer were randomized with a 2×2 factorial design to receive 267 mg/m2 of daily UFT alone Q4W for 12 cycles, 80 mg/m2 of daily S-1 alone for 2 weeks Q3W for 16 cycles, 80 mg/m2 of weekly PAC every 3 or 4 weeks for 1 or 2 cycles, followed by daily UFT Q4W for 9 cycles, or 80 mg/m2 of weekly PAC every 3 or 4 weeks for 1 or 2 cycles, followed by daily S-1 for 2 weeks Q3W for 12 cycles. All patients had previously undergone R0/1 and extended lymph node dissection. Patients were eligible if they had histologically proven gastric adenocarcinoma stage cT3 or T4, N0-2, or M0, were not previously treated with chemotherapy or radiotherapy, had an ECOG PS of 0 to 1, and were able to begin chemotherapy within 14 to 56 days post surgery.
The median follow-up was 1875 days and the final analysis included 1433 patients, with 359 receiving UFT, 364 receiving S-1, 355 receiving PAC and UFT, and 355 receiving PAC and S-1. The primary endpoint was DFS. Overall survival (OS), compliance, and adverse events were secondary endpoints.
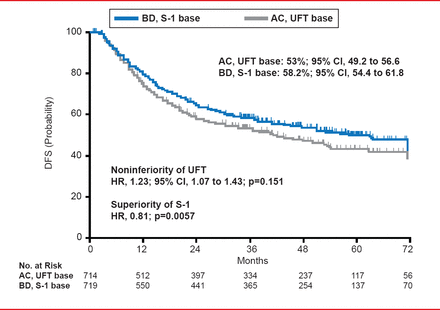
No significant difference in DFS was observed between the UFT and S-1 arms, or the PAC plus UFT and PAC plus S-1 arms. DFS at 3 years occurred in 54% of patients that received UFT or S-1 monotherapy, as compared with 57.2% of patients that received sequential therapy of PAC followed by UFT or S-1 (HR, 0.92; 95% CI, 0.80 to 1.07; p=0.273). However, a number of patients treated with S-1 based therapy (S-1 alone and PAC+S-1) demonstrated 3-year DFS at 58.2%, as compared with 53% of patients that received UFT based therapy (UFT alone and PAC+UFT; Figure 1). This resulted in a HR for noninferiority of UFT of 1.23 (95% CI, 1.07 to 1.43; p=0.151) and an HR of 0.81 for the superiority of S-1 (p=0.0057).
DFS at 3 Years Following UFT or S-1 Monotherapy
Reproduced with permission from K Yoshida, MD, PhD.
Similarly, there was no significant difference in OS between the sequential arms. However, the 5-year OS rate was 60.7% in the S-1 based therapy arm compared with 54.3% in the UFT based therapy arm, resulting in a HR of 1.23 for noninferiority of UFT based therapy (95% CI, 1.04 to 1.44; p=0.161).
The most frequent Grade 3/4 adverse events were neutropenia and anorexia. Neutropenia occurred in 11.4% of patients that received UFT monotherapy, 13.2% of patients that received S-1, 13% of patients that received PAC followed by UFT, and 23.4% of patients that received PAC followed by S-1.
Prof. Yoshida concluded that, in his opinion, data from the SAMIT trial indicated that adjuvant treatment of locally advanced gastric cancer with PAC followed by S-1 is safe and effective. However, although treatment with PAC followed by S-1 did not significantly reduce gastric cancer recurrence, there was a trend for improved DFS. In addition, S-1 treatment was demonstrated to be superior to treatment with UFT.
- © 2013 MD Conference Express®