Summary
For breast cancer patients with a positive sentinel node biopsy, treatment with axillary radiotherapy provides excellent locoregional control that is comparable to axillary lymph node dissection but with significantly less lymphedema. This is the conclusion of the final analysis of the Comparison of Complete Axillary Lymph Node Dissection With Axillary Radiation Therapy in Treating Women With Invasive Breast Cancer trial [AMAROS; NCT00014612; Rutgers EJ et al. J Clin Oncol 2013 (suppl; abstr LBA1001)].
- Breast Cancer
- Radiation Therapy
- Radiology Clinical Trials
- Lymphatic Diseases
- Oncology
For breast cancer patients with a positive sentinel node biopsy (SNB), treatment with axillary radiotherapy (AxRT) provides excellent locoregional control that is comparable to axillary lymph node dissection (ALND) but with significantly less lymphedema.
This is the conclusion of the final analysis of the Comparison of Complete Axillary Lymph Node Dissection With Axillary Radiation Therapy in Treating Women With Invasive Breast Cancer trial [AMAROS; NCT00014612; Rutgers EJ et al. J Clin Oncol 2013 (suppl; abstr LBA1001)] by lead investigator Emiel J. T. Rutgers, MD, Netherlands Cancer Institute, Amsterdam, The Netherlands.
The Phase 3 AMAROS trial was designed to demonstrate the noninferiority of AxRT compared with ALND on 5-year axillary recurrence rate in patients with cT1-2N0 primary breast cancer. Between 2001 and 2010, 4806 patients were enrolled in the trial and, after SNB-positive identification, 1425 were randomized to ALND (n=744) or AxRT (n=681), forming an intention-to-treat (ITT) sample. Patients of any age were eligible for study enrollment, along with those with invasive breast cancer of 0.5 to 5.0 cm and clinical N0 disease and had undergone either breast-conserving surgery or mastectomy. Patients were excluded from the study if they had multicentric disease, neoadjuvant systemic treatment, previous axillary treatment, or prior malignancy.
In patients treated with AxRT, treatment started <12 weeks after SNB, was delivered to levels I, II, III, and the medial supraclavicular node area, at a dose of 25×2 Gy or equivalent.
Baseline characteristics were comparable between the two treatment arms regarding age, characteristics of the tumor (size, grade, and type), as well as use of systemic chemotherapy. In both arms, ∼82% of the patients underwent breast-conserving surgery and ∼17% underwent mastectomy.
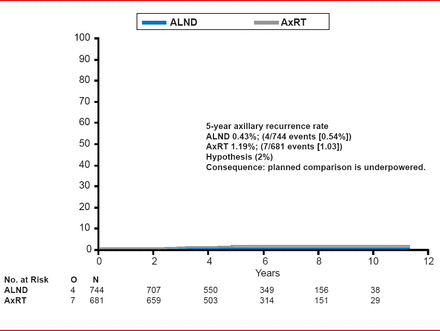
Based on an ITT analysis, the study found that at a median follow-up of 6.1 years the 5-year axillary recurrence rate was 0.54% (4/744 events) for the patients in the ALND arm and 1.03% (7/681 events) in the AxRT arm after a positive SNB (Figure 1). According to Prof. Rutgers, this axillary recurrence rate was far less than anticipated which resulted in the study being underpowered for the planned noninferiority comparison.
The study also looked at secondary endpoints of overall survival (OS) and disease-free survival (DFS), as well as safety (ie, lymphedema, shoulder function, and quality of life). No significant differences were found in the ITT group between ALND and AxRT in OS (92.9% vs 92.1%, respectively; HR, 1.17; 95% CI, 0.85 to 1.62; p=0.34), nor in DFS (HR, 1.17; 95% CI, 0.93 to 1.51; p=0.18).
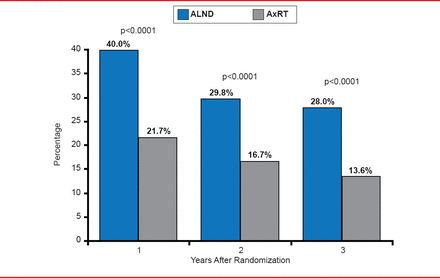
However, the study did find significant differences in the incidence of lymphedema with patients treated with AxRT at half the risk of lymphedema compared with ALND. At 1 year, lymphedema-related symptoms were observed in 40.0 of ALND patients and 21.7% of AxRT patients (p<0.0001) and at 5 years in 28.0% and 13.6% of patients, respectively (p<0.0001; Figure 2).
Axillary Recurrence Rate
Reproduced with permission from EJT Rutgers, MD.
The other measures of safety were comparable between the two arms, with a nonsignificant trend toward more impairment in early shoulder movement after AxRT. No differences in quality of life were found, except for a trend in reduced swelling with AxRT and improved movement with ALND.
Lymphedema: Clinical Observation and/or Treatment
Reproduced with permission from EJT Rutgers, MD.
Based on these results, Prof. Rutgers concluded that AxRT can be considered standard treatment for patients with a positive SNB, offering considerably less lymphedema and comparable locoregional breast cancer control with ALND.
- © 2013 MD Conference Express®