Summary
In a head-to-head comparison, cetuximab (an epidermal growth factor receptor inhibitor) combined with first-line leucovorin/5-fluorouracil/irinotecan (FOLFIRI) chemotherapy improved overall survival relative to bevacizumab (an angiogenesis inhibitor) plus FOLFIRI in patients with KRAS wild-type metastatic colorectal cancer. This article presents the results of the 5-FU, Folinic Acid and Irinotecan Plus Cetuximab Versus FOLFIRI Plus Bevacizumab in First-Line Treatment Colorectal Cancer study, [FIRE-3; NCT00433927; Heinemann V et al. J Clin Oncol 2013 (suppl; abstr LBA3506)].
- Gastrointestinal Cancers Clinical Trials
- Gastrointestinal Cancers
- Oncology
- Oncology Clinical Trials
In a head-to-head comparison, cetuximab (CET; an epidermal growth factor receptor inhibitor) combined with first-line leucovorin/5-fluorouracil/irinotecan (FOLFIRI) chemotherapy improved overall survival (OS) relative to bevacizumab (BEV; an angiogenesis inhibitor) plus FOLFIRI in patients with KRAS wild-type metastatic colorectal cancer (mCRC). Sebastian Stintzing, MD, Ludwig-Maximilian-University of Munich, Munich, Germany, presented the results of the 5-FU, Folinic Acid and Irinotecan (FOLFIRI) Plus Cetuximab Versus FOLFIRI Plus Bevacizumab in First-Line Treatment Colorectal Cancer (CRC) study, [FIRE-3; NCT00433927; Heinemann V et al. J Clin Oncol 2013 (suppl; abstr LBA3506)].
FIRE-3 was a randomized, Phase 3, multicenter trial conducted at 150 centers in Germany and Austria to compare the efficacy of FOLFIRI Q2W (Tournigand regimen) plus CET (400 mg/m2 Day 1, followed by 250 mg/m2 weekly; n=297) or the FOLFIRI regimen plus BEV (5 mg/kg Q2W; n=295). Participants were adults (aged ≥18 years) with histologically confirmed diagnosis of mCRC KRAS wild type and ECOG-PS 0 to 2. Prior adjuvant chemotherapy was permitted if completed >6 months before study start.
The primary study endpoint was overall response rate (ORR) measured by modified RECIST v1.0. Secondary endpoints included progression-free survival (PFS), OS, time to failure of strategy, tumor volume changes, and safety and tolerability.
In total, 752 patients were enrolled in the study, of which 592 were KRAS wild type and formed the intention-to-treat (ITT) population. For the response analysis, a second population was predefined to include patients who received ≥3 cycles of chemotherapy and one computed tomography (CT) scan after baseline (n=526). Study participants were 66% men, median age 64 years; 98% were ECOG PS 0 to 1. There was no significant difference in tumor subtype characteristics, the number of metastatic sites or prior types of treatment between the two groups; ∼31% of patients in both groups had liver metastasis only. Treatment duration was similar, but patients in the BEV arm received a median of two more cycles than those in the CET arm (p=0.014).
Within the ITT population, there was no significant difference in ORR (CET 62% vs BEV 58%; OR, 1.18; 95% CI, 0.85 to 1.64; p=0.183). The primary endpoint of the study has therefore not been met. However, in the 526 patients assessable for response, the ORR for CET was significantly higher than with BEV (72.2% vs 63.1%; OR, 1.52; 95% CI, 1.05 to 2.19; p=0.017).
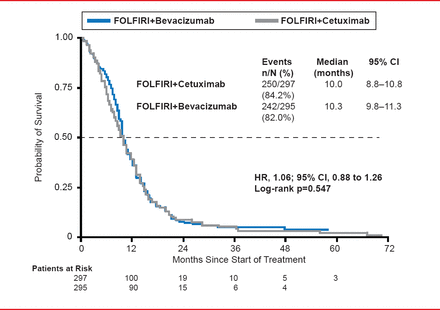
In the ITT population, more patients receiving CET had a complete response, while more patients receiving BEV had stable disease. Median PFS did not differ between the CET (10.0 months) and BEV (10.3 months) treatment groups (HR, 1.06; 95% CI, 0.88 to 1.26; p=0.547; Figure 1).
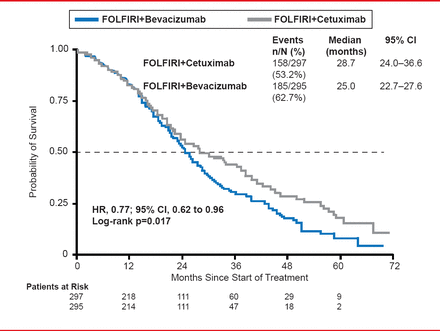
However, OS was significantly prolonged in the CET arm (28.7 months) compared with the BEV arm (25.0 months; HR, 0.77; 95% CI, 0.62 to 0.96; p=0.017; Figure 2).
An exploratory subgroup analysis (age, gender, number of metastatic sites, liver limited disease, and leukocyte counts) favored FOLFIRI plus CET for OS. There were no significant differences in hematological toxicities between treatment arms. Nonhematologic toxicities were comparable for ≥Grade 3, but for any grade toxicity, hand-foot syndrome was more common in the CET arm while nausea and vomiting were more frequent with BEV. Sixty-day mortality was low in both arms (1.01% for CET vs 2.71% for BEV).
Progression-Free Survival
Reproduced with permission from S Stintzing, MD.
In explaining the possible reason for the differences in OS, Prof. Stintzing pointed to the CRYSTAL trial, which showed that tumor size reduction is more predictive of OS than PFS [Mansmann UR et al. J Clin Oncol 2013 (suppl; abstr 3630)]. There is currently an ongoing independent review of the FIRE-3 CT scans to assess tumor volume changes.
Overall Survival
Reproduced with permission from S Stintzing, MD.
- © 2013 MD Conference Express®