Summary
Results from the Efficacy and Safety Study of OncoVEX Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Compared to GM-CSF in Melanoma [OPTiM; NCT00769704; Andtbacka RHI et al. J Clin Oncol 2013 (suppl; abstr LBA9008)] showed that a genetically modified version of herpes simplex virus type 1 (talimogene laherparepvec), is safe and improves durable response rate in patients with unresectable stage IIIB-IV melanoma.
- Soft Tissue Cancers Clinical Trials Genomics
- Soft Tissue Cancers
- Oncology Clinical Trials
- Oncology Genomics
- Oncology
Results from the Efficacy and Safety Study of OncoVEX Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Compared to GM-CSF in Melanoma [OPTiM; NCT00769704; Andtbacka RHI et al. J Clin Oncol 2013 (suppl; abstr LBA9008)] reported by Robert H. I. Andtbacka, MD, University of Utah, Salt Lake City, Utah, USA, showed that a genetically modified version of herpes simplex virus type 1 (talimogene laherparepvec [T-VEC]), is safe and improves durable response rate (DRR) in patients with unresectable stage IIIB-IV melanoma.
T-VEC is an oncolytic immunotherapy derived from herpes simplex virus type-1 designed to selectively replicate within tumors and to produce GM-CSF to enhance systemic antitumor immune responses. OPTiM is a randomized, Phase 3 trial of T-VEC or GM-CSF in patients with unresected melanoma with regional or distant metastases.
OPTiM enrolled adult patients with injectable unresectable stage IIIB/C or IV melanoma. Subjects were randomized to receive intralesional T-VEC (initially ≤4 mL × 106 plaque forming units [pfu]/mL then after 3 weeks, ≤4 mLx108 pfu/mL Q2W) or subcutaneous GM-CSF (125 μg/m2 for 14 days of every 28-day cycle). T-VEC injection volume was based on lesion size. Patients remained on treatment beyond progression unless clinically significant after 24 weeks. The primary study endpoint was DRR, defined as objective complete response (CR) or partial response (PR) lasting for at least 6 months and beginning within 12 months of treatment. Secondary endpoints included overall survival (OS), objective overall response rate (ORR), time-to-treatment failure (TTF) and safety. Responses were per modified World Health Organization criteria by blinded central review.
The intention-to-treat population (57% men; 51% <65 years) comprised 295 subjects randomized to T-VEC and 141 randomized to GM-CSF. About two thirds of the study participants were stage IV (IVM1a 27%, IVM1b 21%, IVM1c 22%) the remainder were stage IIIB/C. T-VEC patients were treated for a median of 23 weeks (range, 0.1 to 78.9) versus 10 weeks (range, 0.6 to 72) for patients receiving GM-CSF.
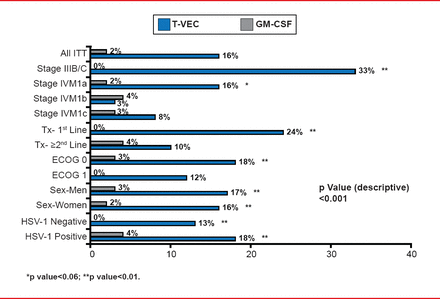
The DRR for T-VEC was 16.3% versus 2.1% with GM-CSF (unadjusted OR, 8.9; 95% CI, 2.7 to 29.2; p<0.0001). The ORR with T-VEC was 26.4% (95% CI, 21.4 to 31.5) with 10.8% CR and 15.6% PR compared with an ORR of 5.7% (95% CI, 1.9 to 9.5) with 0.7% CR and 5.0% PR for GM-CSF. T-VEC was associated with an improvement in DRR in all of the major planned subset analyses, particularly in patients with nonvisceral disease and among patients for whom T-VEC was used as first-line therapy (Figure 1).
Durable Response Rate by Key Covariates
GM-CSF=granulocyte-macrophage colony-stimulating factor; HSV=herpes simplex virus; ITT=intention to treat; T-VEC=talimogene laherparepvec.
Median TTF was 8.2 months for patients treated with T-VEC versus 2.9 months for GM-CSF-treated patients (HR, 0.42; 95% CI, 0.32 to 0.54; p<0.0001). There was a trend to improved OS with T-VEC.
Eleven patients in the T-VEC arm (3.8%) discontinued the study due to an adverse event (AE) compared with 3 patients (2.4%) in the GM-CSF arm. AEs occurring in >20% of patients with T-VEC were fatigue, chills, pyrexia, nausea, flu-like symptoms, injection-site pain and vomiting. The only Grade 3/4 AE occurring in >2% of patients was cellulitis (2.1%). There were 10 fatalities in the T-VEC arm, but none were treatment related.
T-VEC is the first oncolytic immunotherapy to demonstrate therapeutic benefit against melanoma in a Phase 3 trial and represents a novel potential treatment option for melanoma with regional or distant metastases.
- © 2013 MD Conference Express®