Summary
This article discusses the results of the multicenter, randomized, open-label, controlled, Phase 3 Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Methylated Gene Promoter Status study [CENTRIC; NCT00689221; J Clin Oncol 2013 (suppl; abstr LBA2009)].
- Oncology Clinical Trials
- Radiation Therapy
- Head & Neck Cancers
- Radiology
- Oncology Clinical Trials
- Radiation Therapy
- Oncology
- Head & Neck Cancers
- Radiology
Roger Stupp, MD, University of Zurich, Zurich, Switzerland, discussed the results of the multicenter, randomized, open-label, controlled, Phase 3 Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Methylated Gene Promoter Status study [CENTRIC; NCT00689221; J Clin Oncol 2013 (suppl; abstr LBA2009)].
Cilengitide (CIL) is a cyclic arginine, glycine, and aspartic acid-containing pentapeptide that inhibits αvß3 and αvß5 integrins, which are expressed on glioblastoma cells. The CENTRIC study explored the use of CIL combined with standard treatment comprising temozolomide (TMZ) and radiation therapy (RT) for patients with newly diagnosed glioblastoma and methylated O-6 Methylguanine-DNA Methyltransferase (MGMT) gene promoter. Prior Phase 2 trials had suggested a benefits of CIL doses of 500 and 2000 mg on overall survival (OS) and progression-free survival (PFS), with superior outcome of higher versus lower dosage of CIL (2000 vs 500 mg) and little added toxicity [Reardon DA et al. J Clin Oncol 2008; Nabors LB et al. Cancer 2012]. In comparison with historical controls the enhanced benefits of the combination of CIL added to standard TMZ/RT➝TMZ concomitant and adjuvant TMZ and RT treatment sequence was particularly pronounced in tumors containing a MGMT gene promoter methylation [Stupp R et al. J Clin Oncol 2010].
This pivotal Phase 3 trial was conducted at over 200 sites worldwide. Eligibility criteria for the 545 patients were aged ≥18 years, newly diagnosed and histologically proven glioblastoma, methylated MGMT promoter, ECOG PS 0 to 1, and stable or decreasing use of steroids. A total of 545 patients were randomized to standard treatment [Stupp R et al. N Engl J Med 2005] with TMZ/RT➝TMZ and CIL (2000 mg IV BIW) or standard therapy alone. Maintenance TMZ was given for up to 6 cycles, CIL was to be given until disease progression up to 2 years.
The primary endpoint was OS, secondary endpoints were PFS, safety and tolerability, QT/QTc elevation, population pharmacokinetics, general health and work status, and quality of life. The median follow-up was 29 months.
Baseline characteristics in the intention-to-treat population were similar in terms of median age, male sex, ECOG PS, extent of surgery, recursive portioning analysis class, median weeks to randomization, and use of steroid and seizure medications.
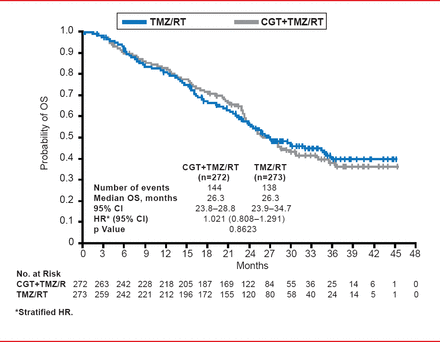
This study failed to meet the primary endpoint as no differences in OS were evident (median treatment OS, 26.3 months; 95% CI, 23.8 to 28.8; median control OS, 26.3 months; 95% CI, 23.9 to 34.7; HR, 1.021; 95% CI, 0.808 to 1.291; p=0.8623; Figure 1). Further analyses of the patients according to parameters including age, ethnicity, region of origin, and extent of surgery did not reveal any significance in terms of OS. Similarly, no differences between the patient groups were apparent for PFS as determined by the individual investigators (median treatment PFS, 13.5 months; 95% CI, 10.8 to 15.9; median control PFS, 10.7 months; 95% CI, 8.1 to 13.3; HR, 0.926; 95% CI, 0.757 to 1.133; p=0.4570) and an overall determination by independent assessors (median treatment OS, 10.6 months; 95% CI, 8.2 to 13.4; median control PFS, 7.9 months; 95% CI, 5.9 to 12.5; HR, 0.918; 95% CI, 0.750 to 1.124; p=0.4102).
Toxicity in both arms was mainly related to TMZ and RT. The marginally increased incidence of pulmonary embolism in the CIL-treated patients (12 vs 5 patients) was not considered clinically relevant. Other adverse events in the two study arms were similar in the two treatment arms.
Overall Survival: Intent to Treat
Reproduced with permission from R Stupp, MD.
The researchers concluded that CIL applied with the standard therapeutic combination of TMZ and RT did not prolong survival, with no patient subgroup exhibiting a clinical benefit. No new safety concerns were evident.
- © 2013 MD Conference Express®