Summary
Ten years of adjuvant tamoxifen is superior to 5 years in reducing the rates of late recurrence and death in women with estrogen receptor (ER)-positive breast cancer. Breast cancer mortality is reduced by about one third over 15 years when women with ER-positive tumors are treated with 5 years of adjuvant tamoxifen. The effect of an additional 5 years of treatment on breast cancer recurrence and death was examined in the randomized Phase 3 Adjuvant Tamoxifen: To Offer More? study [aTTom; ISRCTN17222211; Gray RG et al. J Clin Oncol 2013 (suppl; abstr 5)]
- Breast Cancer Clinical Trials
- Adjuvant/Neoadjuvant Therapy
- Oncology
Ten years of adjuvant tamoxifen is superior to 5 years in reducing the rates of late recurrence and death in women with estrogen receptor (ER)-positive breast cancer.
Breast cancer mortality is reduced by about one third over 15 years when women with ER-positive tumors are treated with 5 years of adjuvant tamoxifen. The effect of an additional 5 years of treatment on breast cancer recurrence and death was examined in the randomized Phase 3 Adjuvant Tamoxifen: To Offer More? study [aTTom; ISRCTN17222211; Gray RG et al. J Clin Oncol 2013 (suppl; abstr 5)], which was presented by Richard G. Gray, MSc, University of Oxford, Oxford, United Kingdom.
In aTTom, 6953 women in the United Kingdom who had been taking tamoxifen for 5 years were randomized to continuing treatment for an additional 5 years or stopping treatment. During the 15-year enrollment period, ER status was tested in only ∼40% of the women; ER status in the remaining 60% was unknown. About 75% of the women assigned to continue tamoxifen had continued taking it for the 5 extra years.
Ten years of tamoxifen reduced breast cancer recurrence by 15% (580 recurrences with allocation to 10 years vs 672 with allocation to 5 years; p=0.003). The number of breast cancer deaths was reduced from 452 with assignment to 5 years to 404 with assignment to 10 years of tamoxifen, a 12% relative reduction in risk (p=0.06). Treatment allocation had little effect on either recurrence rates or death rates from 5 to 9 years after diagnosis but the benefit of longer treatment became evident in the second decade after diagnosis, said Prof. Gray. The relative reduction in the risk of death with assignment to 10 years of tamoxifen increased to 21% in Years 10 to 14 after diagnosis, and to 25% in Year 15 of follow-up and later. These numbers may be underestimates of the true effect of prolonged tamoxifen therapy due to the incluse of the majority of patients with unkown ER status.
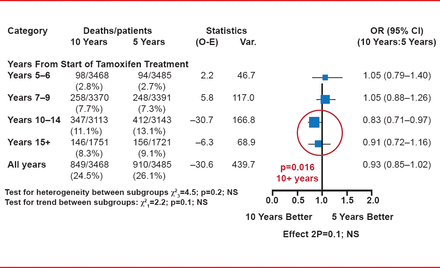
Extending the use of tamoxifen increased the risk of endometrial cancer. There were 102 (2.9%) versus 45 (1.3%) endometrial cancers, with a rate ratio (RR) of 2.20 (p<0.0001), with 37 (1.1%) versus 20 (0.6%) endometrial cancer deaths (RR, 1.83; p=0.02). There were no significant differences in the rates of death without recurrence or all-cause mortality between the two groups, although a significant difference in overall survival in favor of 10 years of tamoxifen emerged from Year 10 onward (p=0.016; Figure 1).
Overall Survival by Treatment and Year of Follow-Up
Reproduced with permission from RG Gray, MSc.
The findings from aTTom complement and confirm those from the recently published international study, Adjuvant Tamoxifen: Longer Against Shorter [ATLAS; Davies C et al. Lancet 2013]. The combined data of aTTom and ATLAS with a total of 17,477 enrolled patients show a significant 15% reduction in breast cancer mortality overall (p=0.001) and an additional 25% reduction in breast cancer mortality 10 years and beyond with 10 years of tamoxifen compared with 5 years of treatment (p=0.00004; Table 1).
Prof. Gray estimates that compared with taking no tamoxifen, 10 years of tamoxifen reduces breast cancer death rate by one third in the first 10 years after diagnosis and by half subsequently.
Breast Cancer Mortality: 10 Versus 5 Years
- © 2013 MD Conference Express®