Summary
Nosocomial pneumonia, especially ventilator-associated pneumonia (VAP), is a major cause of morbidity and mortality in the intensive care unit (ICU). This article discusses updates on bacterial resistance relevant to VAP, with a focus on methicillin-resistant Staphylococcus aureus (MRSA), treating MRSA pneumonia in the ICU, the use of tigecycline and doripenem for hospital-acquired pneumonia and VAP, and the potential utility of inhaled antibiotics as an alternative in the treatment of patients with VAP.
- Nursing
- Bacterial Infections
- Pneumonia
- Drug Resistance
- Nursing
- Bacterial Infections
- Pneumonia
- Pulmonary & Critical Care
- Drug Resistance
Nosocomial pneumonia, especially ventilator-associated pneumonia (VAP), is a major cause of morbidity and mortality in the intensive care unit (ICU).
Mark L. Metersky, MD, University of Connecticut School of Medicine, Farmington, Connecticut, USA, provided an update on bacterial resistance relevant to VAP, with a focus on methicillin-resistant Staphylococcus aureus (MRSA). Vancomycin-resistant S. aureus is rare (limited to case reports) but the prevalence of vancomycin-intermediate S. aureus (VISA) is probably increasing. Heteroresistant VISA (hVISA) is challenging to detect; using population analysis profiling area under the curve (AUC) as a reference method, the hVISA phenotype can be detected for strains of S. aureus with vancomycin minimum inhibitory concentration (MIC) levels as low as 0.5 μg/mL [Howden BP et al. Clin Microbiol Rev 2010; Musta AC et al. J Clin Microbiol 2009].
Vancomycin MIC “creep” refers to an increase in the frequency of MICs approaching 2 μg/mL, considered to be the cutoff for vancomycin sensitivity. The significance of the MIC creep is not clear, as studies assessing the impact of vancomycin treatment on response have produced variable results. Regional variation in the contribution of MRSA to S. aureus VAP is marked. In the United States and Europe, the prevalence of MRSA seems to have stabilized, although at various levels depending upon the country studied.
Gram-negative resistance in VAP continues to increase, but like MRSA, the prevalence across the world varies widely. For example, Acinetobacter sp. cause approximately 5% of VAP in the United States [Jones RN. Clin Infect Dis 2010] and ∼35% in Asia)[Chung DR et al. Am J Respir Crit Care 2011]. This organism is typically multi-drug resistant. Klebsiella pneumoniae is a Gram-negative organism that is increasingly resistant to carbapenems; several other Gram-negative Enterobacteriaceae and non-Enterobacteriaceae also produce carbapenemases. In vitro ertapenem resistance suggests K. pneumoniae carbapenemase with resulting imipenem and meropenem resistance despite in vitro susceptibility, said Dr. Metersky.
Antibiotic stewardship is necessary to help limit the development of resistant strains in the hospital. The elements of stewardship include treating infection and not treating colonization, determining the infecting organism, initial appropriate antibiotic therapy at a sufficient dose, deescalation, and avoiding excessive length of therapy.
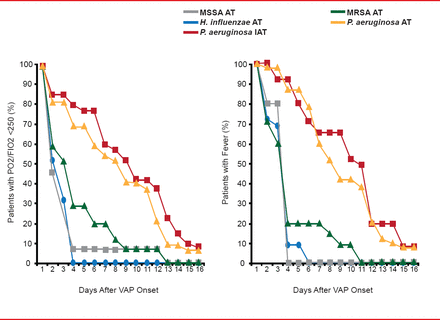
Michael S. Niederman, MD, Winthrop University Hospital, Mineola, New York, USA, discussed treating MRSA pneumonia in the ICU, noting that resistance is a risk factor for mortality. Even with appropriate therapy with vancomycin, mortality for those with MRSA VAP was 48% in one series of 75 cases, which was double the risk of mortality compared with controls [Rello J et al. Crit Care Med 2005]. In a subset of patients who received continuous-infusion vancomycin, however, the mortality rate declined to half compared with those treated with intermittent vancomycin, which suggests that attributable mortality of MRSA VAP could be lowered with better therapies. Other studies show longer length of stay [Shorr AF et al. Crit Care Med 2006] and slower resolution of MRSA VAP than other forms of pneumonia, despite appropriate treatment (Figure 1) [Vidaur L et al. Chest 2008].
Combination therapy is needed to optimize treatment of Gram-negative bacteria in VAP, and usually requires a β-lactam antibiotic with an aminoglycoside. In 2013, MRSA must be considered when selecting therapy in virtually every patient on a ventilator who develops nosocomial pneumonia, said Dr. Niederman. With rising MIC levels for vancomycin, optimizing drug dosing is essential, although even optimization may not lead to good outcomes if the vancomycin MIC is >1 μg/mL, and aggressive vancomycin dosing may promote nephrotoxicity [Lodise TP et al. Clin Infect Dis 2009].
For proven MRSA VAP, linezolid may offer advantages over optimally dosed vancomycin. In a head-to-head, randomized, double-blind comparison, clinical response at end of study was significantly higher with linezolid than with vancomycin dosed optimally in the treatment of documented MRSA nosocomial pneumonia [Wunderink RG et al. Clin Infect Dis 2012] although 30-day mortality was not different between the two treatment groups (∼15% in each group). One factor that may have prevented detection of a difference in mortality was that clinical failures with vancomycin were allowed salvage therapy with linezolid.
MRSA VAP Is Slow to Clinically Resolve
MRSA=methicillin-resistant Staphylococcus aureus; MSSA=methicillin-sensitive Staphylococcus aureus; VAP=ventilator-associated pneumonia.
Reproduced from Vidaur L et al. Ventilator-Associated Pneumonia: Impact of Organisms on Clinical Resolution and Medical Resources Utilization. Chest 2008;133(3):625. With permission from Elsevier.
Richard Wunderink, MD, Northwestern University, Chicago, Illinois, USA, reviewed data from recent trials of tigecycline and doripenem for hospital-acquired pneumonia (HAP) and VAP. In a randomized comparison of tigecycline and imipenem/cilastatin for the treatment of HAP, the clinical response rate was markedly superior with imipenem in a subset with VAP (47.9% vs 70.1%) and in those with MRSA (40.0% vs 81.8%), Acinetobacter spp (57.1% vs 94.7%), or Pseudomonas aeruginosa (27.3% vs 85.7%) infection [Freire AT et al. Diagn Microbiol Infect Dis 2010].
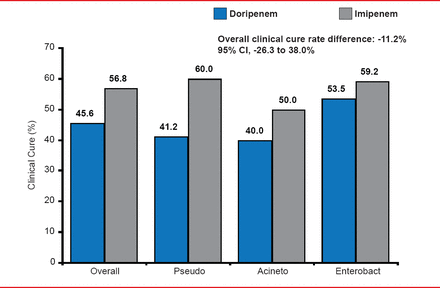
In a randomized trial of 7 days of doripenem versus 10 days of imipenem/cilastatin in patients with VAP, the overall clinical cure rate was better with imipenem/cilastatin (56.8%) than with doripenem (45.6%; Figure 2) [Kollef MH et al. Crit Care 2012]. Inadequate dosing of tigecycline as determined by the fAUC0–24:MIC ratio appears to be responsible for lower clinical and microbiologic responses in VAP patients [Bhavnani SM et al. Antimicrob Agents Chemother 2012]. One lesson is that the pharmacokinetic/pharmacodynamics characteristics in some critically ill patients are different, including an increased creatinine clearance.
In both of the aforementioned studies, the mortality rate was lower with imipenem/cilastatin but differences did not emerge until after the study drugs were stopped. These data suggest that VAP does have an attributable mortality that tracks with the more subjective clinical response, doesn't usually occur during the time of VAP treatment, and is only revealed when no salvage therapy is available.
In patients with difficult to treat pathogens, minimum bactericidal concentrations may be more relevant than MIC in determining an adequate dose and duration of treatment, said Dr. Wunderink. In some cases, a loading dose may be needed when prolonged infusions are chosen.
Clinical Response in Carbapenem VAP Trial
Antonio Anzueto, MD, University of Texas Health Science Center, San Antonio, Texas, USA, discussed the potential utility of inhaled antibiotics as an alternative in the treatment of patients with VAP. Nebulized antibiotics provide higher pulmonary concentrations than intravenous (IV) administration at the same dose, and are influenced by lung aeration [Elman M et al. Anesthesiology 2002]. Aerosolized antibiotics reduced signs of respiratory infection and the bacterial burden in tracheal aspirates in critically ill intubated patients [Palmer LB et al. Crit Care Med 2008].
Pulmonary delivery of aerosolized amikacin exceeded the MIC levels in the alveolar epithelial lining fluid for Gram-negative microorganisms usually responsible for VAP [Luyt CE et al. Crit Care 2009].
In a Phase 2 trial, nebulized ceftazidime and amikacin had similar success as IV administration in the treatment of patients with P. aeruginosa VAP (n=40), and acquisition of per-treatment antibiotic resistance was observed only in the IV group [Lu Q et al. Am J Respir Crit Care Med 2011].
An investigational delivery system for amikacin achieved tracheal aspirate amikacin maximum concentration and a ratio of area under the aspirate concentration-time curve in 50% of patients with dosing every 12 hours in a Phase 2 randomized, placebo-controlled study of 69 mechanically ventilated patients with Gram-negative pneumonia [Niederman MS et al. Intensive Care Med 2012], and warrants further clinical evaluation.
- © 2013 MD Conference Express®