Summary
Venous thromboembolism is responsible for the hospitalization of more than 250,000 Americans annually and represents a significant risk for morbidity and mortality [Jaff MR et al. Circulation 2011]. Approximately 5% of pulmonary embolisms (PEs) are considered massive (systolic blood pressure <90 mm Hg or a =40 mm Hg drop in BP for >15 minutes + signs of hypoperfusion). Although not always easy to do, distinguishing between massive and nonmassive events is important. This article discusses the management of massive PE, the use of IVC filters in patients with PE, the use of new anticoagulants compare with older therapies, and the the practical use of biomarkers and scoring systems for patients with PE.
- Pulmonary Genomics
- Pulmonary Guidelines
- Thromboembolic Disease
- Thrombotic Disorders
- Interventional Radiology
- Pulmonary Genomics
- Pulmonary & Critical Care
- Pulmonary Guidelines
- Thromboembolic Disease
- Thrombotic Disorders
- Interventional Radiology
Venous thromboembolism is responsible for the hospitalization of more than 250,000 Americans annually and represents a significant risk for morbidity and mortality [Jaff MR et al. Circulation 2011].
Approximately 5% of pulmonary embolisms (PEs) are considered massive (systolic blood pressure [SBP] <90 mm Hg or a ≥40 mm Hg drop in BP for >15 minutes + signs of hypoperfusion). Although not always easy to do, distinguishing between massive and nonmassive events is important as patients with massive PE are more likely to experience a recurrence within 90 days (12.6% vs 7.6%, for nonmassive PE; p<0.001) and they have a higher mortality rate (52.4% vs 14.7%; p<0.001) primarily due to recurrence [Kucher N et al. Circulation 2006]. Richard N. Channick, MD, Massachusetts General Hospital, Boston, Massachusetts, USA, discussed the management of massive PE.
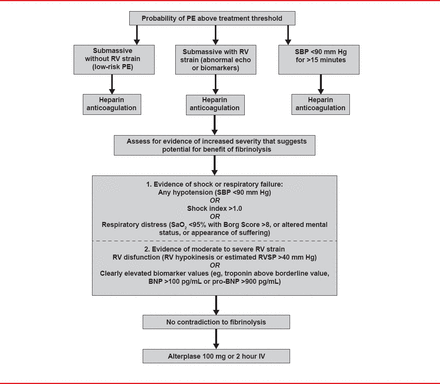
Unless contraindicated, fibrinolysis is the treatment of choice (Figure 1) [Jaff MR et al. Circulation 2011]. For patients with massive PE who remain unstable after receiving fibrinolysis or in whom fibrinolysis is contraindicated, current guidelines suggest consideration of either catheter or surgical embolectomy. Either option may also be considered for patients with sub-massive acute PE [Jaff MR et al. Circulation 2011].
Suggested Treatment Algorithm for Use of Fibrinolytics to Treat Acute PE
BNP=brain natriuretic peptide PE=pulmonary embolism; RV=right ventricular; RVSP=right ventricular systolic pressure; SBP=systolic blood pressure.
Reprinted from Jaff MR et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension: A Scientific Statement From the American Heart Association. Circulation 2011;123(16):1788–1830. With permission from Lipincott, Williams and Wilkins.
Dr. Channick noted that no precise algorithms exist for determining which patients will do better with systemic lysis versus catheter-directed thrombolysis versus surgery. Patient selection should be based on clinical and hemodynamic status, degree of right ventricular (RV) dysfunction, and contraindications to systemic lysis.
Although the evidence indicates that the use of inferior vena cava (IVC) filters leads to a reduction in the incidence of PE this benefit appears to be counterbalanced by an excess of deep-vein thrombosis (DVT) [Decousus H et al. N Engl J Med 1998; PREPIC Study Group Circulation 2005]. In addition, data on the effect of these filters on mortality is contradictory [Proctor MC, Greenfield LJ. Cardiovasc Surgery 1997; Spencer FA et al. Arch Intern Med 2010]. Todd M. Bull, MD, University of Colorado, Aurora, Colorado, USA, discussed some of the data supporting the use of IVC filters in patients with PE.
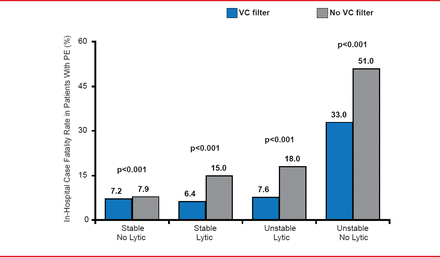
Results from a large (>2,000,000 patients) retrospective study investigating the use of IVC filters in patients with PE showed a significantly lower in-hospital case fatality rate among stable patients without DVT who received a filter (7.2% vs 7.9%; p<0.0001). The case fatality rate was also lower in the small percentage of stable patients (1.4%) who were also receiving thrombolytic therapy (6.4% vs 15% with no thrombolysis; p<0.0001). For unstable patients the in-hospital fatality rate was lower in patients who received a filter regardless of whether they received thrombolytic therapy (7.6% vs 18% and 33% vs 51%, with/without thrombolytic therapy, respectively; both p<0.0001; Figure 2) [Stein PD et al. Am J Med 2012]. The investigators concluded that IVC filters can be considered for patients with PE who are receiving thrombolytic therapy and for unstable patients who may not be candidates for thrombolytic therapy.
Effects of Vena Cava Filters on Case Fatality
PE=pulmonary embolism; VC=vena cava.
Reproduced from Stein PD et al. Impact of Vena Cava Filters on In-Hospital Case Fatality Rate from Pulmonary Embolism. Am J Med 2012. With permission from Elsevier.
Although current American College of Chest Physicians guidelines state that IVC filters should not be used in addition to anticoagulation, they are recommended for patients in whom anticoagulation is contraindicated. If an IVC filter is inserted as an alternative to anticoagulation, it should be followed by a conventional course of anticoagulant therapy if the risk of bleeding resolves [Kearon C et al. Chest 2012]. The American Heart Association guidelines are similar in that they recommend the use of IVC filters in patients with a contraindication to anticoagulation and in the case of failure of anticoagulation. IVC filters may also be considered for patients with very poor cardiopulmonary reserve but should not be routinely used as an adjunct to anticoagulation or lysis [Jaffe MR et al. Circulation 2011].
Dr. Bull commented that while it is clear the IVC filters are appropriate when anticoagulation is not possible there remains a lack of good data for other scenarios such as in cases where anticoagulation has failed, in patients with poor cardiopulmonary reserve, as prophylaxis in high-risk patients and in the case of chronic thromboembolic pulmonary hypertension. The National Institute of Health is currently considering a study investigating the use of IVC filters in people with stable high and moderate risk for PE.
David J. Kuter, MD, PhD, Massachusetts General Hospital, Boston, Massachusetts, USA, discussed how some of the new anticoagulants compare with older therapies such as warfarin and heparin.
Unlike warfarin and heparin, which act at multiple sites in the coagulation cascade, some of the new anticoagulants act directly on thrombin either by irreversibly (lepirudin) or reversibly (bivalirudin) binding to the fibrinogen binding exosite and the active site pocket or by reversibly binding to only the active site (argatroban and dabigatran etexilate). Dabigatran etexilate is a twice-daily oral therapy with a long half-life (12 to 17 hours) that is cleared via the kidney (80%). Although not yet approved for this indication, dabigatran etexilate has been shown to be noninferior to warfarin in the management of DVT/PE, with a similar safety profile. Unlike warfarin, dabigatran does not require laboratory monitoring [Schulman S et al. N Engl J Med 2009].
Other new anticoagulants act on Factor Xa. Rivaroxaban is a once daily, oral direct inhibitor of Factor Xa, with a flat dose response curve, and a half-life of 5 to 9 hours. Clearance is via the kidneys (51%) [Kubitza D et al. Clin Pharmacol Ther 2005]. In most situations, rivaroxaban does not require monitoring. It is approved for the prophylaxis of DVT, which may go on to PE in patients undergoing hip or knee replacement surgery, to reduce the risk of stroke and systemic embolism in patients with non-valvularatrial fibrillation, and for the treatment of DVT/PE. It is noninferior to standard therapy for the treatment of PE and DVT with a potentially improved benefit–risk profile [EINSTEIN Investigators. N Engl J Med 2010; Einstein-PE Investigators. N Engl J Med 2012]. Apixiban is a twice-daily oral Factor Xa inhibitor that has mostly hepatic clearance (only 25% renal); though promising, it has not yet been approved for PE/DVT.
Dr. Kuter noted that warfarin and heparin still work well, although heparin is probably more effective in acute situations because of its additional anticoagulation mechanism. He suggested considering the newer oral agents for stable patients who are ready for discharge to treat both the DVT and PE (Table 1).
Comparative Features of Warfarin and New Oral Anticoagulants
Victor F. Tapson, MD, Duke University Medical Center, Durham, North Carolina, USA, discussed the practical use of biomarkers and scoring systems for patients with PE.
All acute PE patients should be risk stratified, most importantly to determine who is at high risk for adverse events, and thus might benefit from escalation of therapy. However, despite the large body of research on biomarkers (ie, brain natriuretic peptide, troponin, and heart-type fatty acid binding protein), none, alone is sufficient for risk stratification, due to the limitations in the studies that have assessed them. These limitations include the absence of a standardized definition of RV dysfunction by echocardiogram, the lack of cardiac biomarker test standardization (different assays and thresholds for positive) and inadequate leg DVT assessment. Several scoring indices are available to support the data from the biomarkers but again none alone is sufficient. Standardized definitions and approaches are needed.
- © 2013 MD Conference Express®