Summary
A once-daily inhaled dual bronchodilator consisting of a long-acting β2-agonist and a long-acting muscarinic antagonist significantly improved self-reported shortness of breath and lung function compared with placebo and tiotropium in patients with moderate to severe chronic obstructive pulmonary disease. This article discusses results of the Effect of QVA149 on Dyspnea in Patients With Chronic Obstructive Pulmonary Disease study [BLAZE; NCT01490125], a multicenter, randomized, blinded, double-dummy, placebo-controlled, 3-period, crossover trial.
- Chronic Obstructive Pulmonary Disease Pulmonary Clinical Trials
- Chronic Obstructive Pulmonary Disease
- Pulmonary & Critical Care
- Pulmonary Clinical Trials
A once-daily inhaled dual bronchodilator consisting of a long-acting β2-agonist and a long-acting muscarinic antagonist (QVA149) significantly improved self-reported shortness of breath and lung function compared with placebo and tiotropium in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Donald A. Mahler, MD, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA, presented results of the Effect of QVA149 on Dyspnea in Patients With Chronic Obstructive Pulmonary Disease study [BLAZE; NCT01490125], a multicenter, randomized, blinded, double-dummy, placebo-controlled, 3-period, crossover trial.
Dyspnea in COPD is not always controlled adequately by bronchodilator monotherapy, providing the rationale for combining two bronchodilators with different mechanisms of action [Rabe KF et al. Am J Respir Crit Care Med 2007; Vestbo J et al. Am J Respir Crit Care Med 2013]. QVA149 is an investigational fixed-dose combination of indacaterol maleate 110 μg and glycopyrronium bromide 50 μg. QVA149 had previously demonstrated improvements in dyspnea versus its individual components, tiotropium, and salmeterol/fluticasone using the interviewer-based Transition Dyspnea Index (TDI) [Bateman ED et al. Eur Respir J 2013; Vogelmeier C et al. Respir Med 2013].
In BLAZE, patients with moderate to severe COPD (n=246) were randomized to once-daily QVA149, tiotropium 18 μg, or placebo. Patients were current or former smokers, had a Modified Medical Research Council scale of >2 at screening, a postbronchodilator forced expiratory volume in 1 second (FEV1) ≥30 and <80% predicted, and FEV1/forced vital capacity <0.7. There was a 2-week washout between crossover periods.
The primary objective was superiority of QVA149 versus placebo on the improvement in patient-reported levels of breathlessness during daily activities using the Self-Administered Computerized (SAC) version of the Baseline Dyspnea Index (BDI)/TDI after 6 weeks. The SAC version of the BDI/TDI was developed as a tool to provide direct patient-reported ratings of dyspnea and to provide a standard method to reduce the potential bias with an interviewer [Mahler DA et al. COPD 2004]. The secondary objective was superiority of QVA149 versus tiotropium on the same endpoint.
Other secondary objectives included evaluation for lung function by FEV1 area under the curve from 0 to 4 hours (AUC0–4h) and rescue medication use over 6 weeks.
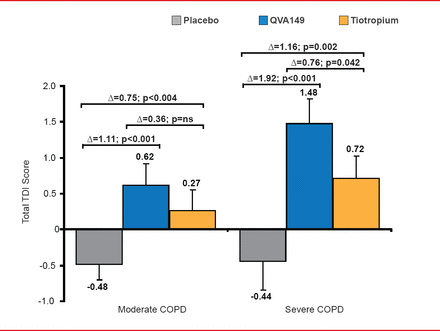
After 6 weeks of treatment, QVA149 significantly improved patient self-reported shortness of breath during daily activities versus placebo (Δ=1.37; p<0.001) and versus tiotropium (Δ=0.49; p=0.021). Significantly more moderate COPD patients achieved ≥1 point TDI total score improvement on QVA149 (35.9%) versus placebo (18.1%; p<0.001) and tiotropium (24.4%; p=0.012). Subgroup analysis showed that the improvement in the SAC TDI with QVA149 was more pronounced in patients with severe COPD (Figure 1).
Subgroup Analysis by COPD Severity
COPD=chronic obstructive pulmonary disease; LS=least squares; NS=nonsignificant; SE=standard error; TDI=Transition Dyspnea Index.
Reproduced with permission from DA Mahler, MD.
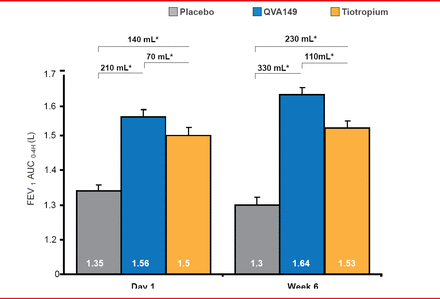
QVA149 produced significant and clinically meaningful improvements in FEV1 AUC0–4h versus placebo and versus tiotropium on Day 1 and Week 6 (p<0.001 for both comparisons; Figure 2).
FEV1 Area Under the Curve
AUC0–4h=area under the curve from 0 to 4 hours; FEV1=forced expiratory volume in 1 second; LS=least squares; SE=standard error.
Reproduced with permission from DA Mahler, MD.
Rescue medication use over 6 weeks was significantly lower with QVA149 versus placebo (−1.43 puffs/day; p<0.001) and tiotropium (−0.45 puffs/day; p=0.002).
QVA149 was well tolerated. The most frequent adverse events (COPD, nasopharyngitis, cough, and dyspnea) occurred with similar frequency to that of placebo and tiotropium.
Results from BLAZE add to the evidence that improved lung function with once-daily QVA149 translates into greater relief of breathlessness and improved patient-reported outcomes, concluded Dr. Mahler.
- © 2013 MD Conference Express®