Summary
This article presents the results of a retrospective study that demonstrated a trend toward additional clinical improvement when spironolactone was added to ambrisentan for the treatment of patients with pulmonary arterial hypertension. This study analyzed data from patients in the double-blind, placebo-controlled Ambrisentan in Patients With Moderate to Severe Pulmonary Arterial Hypertension studies [ARIES-1 and −2; Galiè N et al. Circulation 2008].
- Thromboembolic Disease
- Pulmonary Clinical Trials
- Pulmonary & Critical Care
- Thromboembolic Disease
- Pulmonary Clinical Trials
- Pulmonary & Critical Care
Bradley Maron, MD, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA, presented the results of a retrospective study that demonstrated a trend toward additional clinical improvement when spironolactone was added to ambrisentan for the treatment of patients with pulmonary arterial hypertension (PAH).
In patients with PAH, levels of the mineralocorticoid hormone aldosterone are increased in the pulmonary arterial circulation and correlate positively with hemodynamic measures of pulmonary vascular remodeling [Maron BA et al. Eur J Heart Fail 2013]. In addition, results from recent basic and translational models of PAH suggest that hyperaldosteronism modulates a pulmonary vasculopathy by promoting endothelin receptor type-B (ETB) dysfunction in pulmonary endothelial cells [Maron BA et al. Circulation 2012; Maron BA et al. Am J Cardiol 2013. In press], which is required for maintaining normal bioavailable levels of the vasodilator and anti-remodeling molecule nitric oxide. Together, these observations raised the possibility that coupling the mineralocorticoid-receptor antagonist spironolactone with therapies that inhibit the adverse effects of ETA-mediated pulmonary vasoconstriction/remodeling may be a useful therapeutic strategy for patients with PAH.
This study analyzed data from patients in the double-blind, placebo-controlled Ambrisentan in Patients With Moderate to Severe Pulmonary Arterial Hypertension studies [ARIES-1 and −2; Galiè N et al. Circulation 2008] who were randomized to receive placebo or the selective ETA antagonist ambrisentan (10 mg daily) and in whom spironolactone use was reported. The investigators elected to study patients randomized to the maximum dose of ambrisentan because it was at this dose that the greatest benefit on clinical outcome was observed in the ARIES trial. Critiera for inclusion in the spironolactone treatment group was as follows: study drug use for ≥28 days, initiation of spironolactone prior to enrollment or ≤28 days after first ARIES study drug day, and discontinuation of spironolactone >28 days prior to the final ARIES study drug dose. Twenty-one patients in the placebo group (21/132; 15.9%) and 10 patients in the ambrisentan group (10/67; 14.9%) met the spironolactone criteria.
Patients treated with ambrisentan plus spironolactone tended to have increased mean pulmonary vascular resistance (14.5±6.0 vs 10.8±5.7 Wood units; p=0.07) and plasma B-type natriuretic peptide (BNP) concentrations (236.7 ng/L; 95% CI, 81.5 to 687.4 vs 131.7 ng/L; 95% CI, 88.0 to 197.2; p=0.24) compared with those treated with ambrisentan alone.
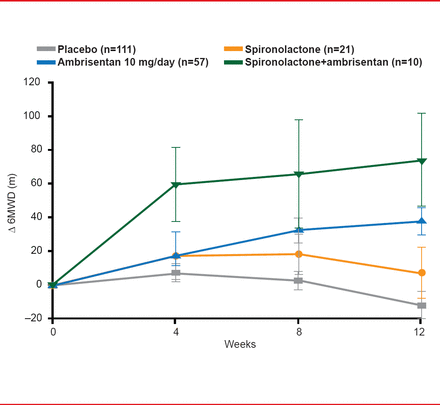
On the primary endpoint of change from baseline in 6-minute walk distance at Week 12, patients treated with ambrisentan plus spironolactone achieved a mean peak mean change of +74.2 meters compared with +38.2 meters for those treated with ambrisentan alone (p=0.11; Figure 1).
A similar trend was observed for the predetermined secondary endpoints: ambrisentan plus spironolactone was associated with a 1.7-fold improvement in BNP levels (p=0.08) compared with ambrisentan alone at Week 12 and a decrease in the geometric BNP mean of 66% compared with 39% decrease for ambrisentan alone at Week 12. World Health Organization functional status improved by ≥1 class in 50.0% of patients treated with ambrisentan plus spironolactone versus 21.6% of placebo-treated patients (p=0.01). Additionally, none of the patients treated with ambrisentan plus spironolactone reached the clinical endpoints of progressive illness, PAH-associated hospitalizations, and/or death versus 5.3% of patients treated with ambrisentan only.
Change From Baseline in the 6-Minute Walk Distance
Reproduced with permission from B Maron, MD.
Limitations of the study include the retrospective design and small sample size. In addition, aldosterone levels were not measured in the ARIES trial nor were potential mechanisms by which aldosterone influenced outcomes. Additionally, the investigators recognized that use of spironolactone may be a marker of more severe PAH and that the clinical response observed in patients treated with ambrisentan plus spironolactone reflects the enhanced therapeutic efficacy of ambrisentan. Nevertheless, these results support the hypothesis that spironolactone and ETA antagonism may be beneficial in PAH, and, overall, results from this study provide evidence to support future clinical trials to characterize the efficacy of aldosterone inhibition in the treatment of PAH.
- © 2013 MD Conference Express®