Summary
In this article, the American Heart Association (AHA) and Heart Rhythm Society (HRS) discussed the implications of guidelines, some of their limitations, and where guidelines fit in the new paradigm of health care. Specific topics include limitations of the current system of guidelines and how they fit with the new paradigm of health care, the impact of the Medicare and Medicaid services, and the disconnect between indicated ICD therapy as outlined in the ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities [Epstein J et al. J Am Coll Cardiol 2008] and CMS reimbursement rules.
- Cardiology Guidelines
- Arrhythmias
- Cardiology Guidelines
- Cardiology & Cardiovascular Medicine
- Arrhythmias
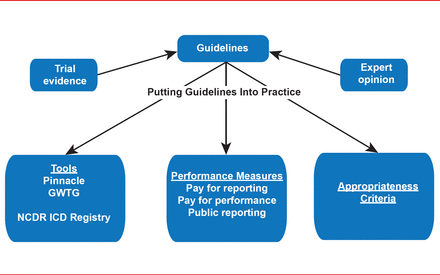
In this special joint session of the American Heart Association (AHA) and Heart Rhythm Society (HRS) speakers discussed the implications of guidelines, some of their limitations, and where guidelines fit in the new paradigm of health care (Figure 1).
Improving Quality
GWTG= Get With the Guidelines; NCDR ICD=National Cardiovascular Data Registry Implantable Cardioverter Defibrillator. Reproduced with permission from M Estes, MD.
Physicians are increasingly hearing about accountability for quality metrics and performance measures and the programs that seek to bridge the gap between the guidelines and practice. N. A. Mark Estes, MD, Tufts Medical Center, Boston, Massachusetts, USA, discussed some of the limitations of the current system of guidelines and how they fit with the new paradigm of health care.
Factors that may prove problematic as we move forward to evidence-based medicine and pay-for-performance are the differences between clinical trial patients and those seen in clinical practice and the fact that <15% of the current guidelines are based on randomized trials [Tricoci P et al. JAMA 2009]. Other factors include the slowness of the current process (the average update takes ∼2 years) and the gap between the number of physicians who say they know the guidelines (95%) and those who treat to goal (18%) [Pearson TA et al. Arch Int Med 2000].
Despite their limitations, Dr. Estes believes that guidelines will become the basis for quality metrics and performance measures as we move forward. In this new paradigm, pay-for-performance is incorporated into healthcare reform and accountability will be measured and reported with registries, databases, and electronic health records serving as the instruments of assessment. The shift will be from a quantity- and procedures-based system to a system based on quality, performance, outcomes, and value. Professional societies will need to take a leadership role in developing the necessary education vehicles and registries.
David E. Haines, MD, Beaumont Health Systems, Royal Oak, Michigan, USA, discussed the impact of the Medicare, Medicaid, Children's Health Insurance Programs; Transparency Reports and Reporting of Physician Ownership or Investment Interests Rule, (the Sunshine Act). Under this rule all payments or transfers of value >$10 made to physicians and teaching hospitals will be posted on the Centers for Medicare & Medicaid Services (CMS) website with data aggregated, downloadable, and searchable. Data collection for the new rule starts August 1, 2013, for reporting to CMS on March 31, 2014 (Table 1).
Examples of Relationships With Industry
The new rule will make possible independent validation of the relationships with industry statements issued by societies, professional organizations, and physicians. However, such a policy may also serve as a disincentive to physician-industry collaboration on new technologies. Given that many of the advances in electrophysiology and pacing are due to a unique collaboration between physicians and industry, Dr. Haines believes that the HRS needs to establish strict ethical standards to protect the credibility of the society and its members. Current policies with respect to writing groups prohibit ownership of equity interests, stocks, or stock options or ownership, partnership, or principal interest in a financially interested enterprise, excluding mutual funds. Members may not have the potential to profit financially from the recommendations of the document. One chair must be free of all “relevant” relationships with industries, and all authors must disclose their relationships with industry for the previous 12 months and update as relationships change.
Guidelines serve several purposes, but primarily they exist to convert the clinical evidence base into clinical instructions. John Camm, MD, St. George's Hospital Medical School, London, United Kingdom, noted that it is not unusual for several different organizations to issue treatment guidelines on the same subject. As an example, he noted that four societies have issued new/updated guidelines on the management of atrial fibrillation (AF) in the past 2 years. National differences are not surprising and are frequent. Differences are seen on several levels ranging from language (eg, softer in Europe vs more directive in the United States) to the basis on which a particular therapy should be selected. By way of example, Prof. Camm discussed how the use of the US-designed CHADS2 scoring system versus the European designed CHA2DS2-VASC scoring affects the treatment recommendation on the choice of an oral anticoagulant in AF. While most differences are not critical, clinicians need to be aware that they exist and understand why these differences may occur.
The 2006 American College of Cardiology (ACC)/AHA/European Society of Cardiology (ESC) Guidelines for the management of patients with ventricular arrhythmias and the prevention for sudden cardiac death [Zipes DP et al. Circulation 2006] provide the only guidance on arrhythmogenic disorders in terms of the use of defibrillators and medical therapy. Silvia G. Priori, MD, PhD, University of Pavia, Pavia, Italy, reviewed some of the current recommendations in need of updating. One of these is the Class I recommendation for an ICD in patients who have survived a cardiac arrest. Prof. Priori suggested that it is important to consider whether the cardiac arrest occurred before initiation of β-blockers. If so, drug therapy may be more appropriate, especially in long QT syndrome Type 1 (LQT1) patients. Another recommendation in need of revision is the Class lib recommendation concerning ICDs as primary prophylaxis in patients with LQT2 and LQT3. Dr. Priori suggested that even though these genetic forms have a worse outcome, other risk factors play a role in the treatment decision. For example, an LQT2 with a QT that is almost normal is at less risk than an LQT1 with a QT of 550.
Hugh Calkins, MD, Johns Hopkins Hospital, Baltimore, Maryland, USA, discussed the disconnect between indicated ICD therapy as outlined in the ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities [Epstein J et al. J Am Coll Cardiol 2008] and CMS reimbursement rules. He told the audience that the HRS hopes to address the gaps by developing a consensus statement in collaboration with the American College of Cardiology Foundation (ACCF) and the AHA [Kusamoto F et al. Submitted]. The statement covers ICD therapy in patients not represented in clinical trials. Topics include ICD implantation
-
▪ in the setting of an abnormal troponin that is not due to a myocardial infarction (MI);
-
▪ within 40 days of an MI;
-
▪ within 90 days of revascularization; and
-
▪ <9 months from the initial diagnosis of nonischemic cardiomyopathy.
Guidelines will continue to provide important information about how new drugs and device therapies and additional clinical evidence for older therapies, affect clinical practice; however, it appears that they are taking on additional significance as we progress to a new healthcare paradigm.
- © 2013 MD Conference Express®