Summary
Patients with heart failure (HF) often remain symptomatic and have a poor prognosis despite treatment with existing therapies [Cleland JG et al. Lancet 2011]. Several new therapeutic options are currently emerging for HF, including direct renin inhibitors, neprilysin inhibitors, selective If channel inhibitors, cardiac myosin activators, vasopressin receptor antagonists, and phosphodiesterase type 5 inhibitors. This article discusses clinical trial data for direct renin inhibitors and neprilysin inhibitors.
- Heart Failure
- Cardiology & Cardiovascular Medicine
- Heart Failure
Patients with heart failure (HF) often remain symptomatic and have a poor prognosis despite treatment with existing therapies [Cleland JG et al. Lancet 2011]. Several new therapeutic options are currently emerging for HF, including direct renin inhibitors, neprilysin inhibitors, selective If channel inhibitors, cardiac myosin activators, vasopressin receptor antagonists, and phosphodiesterase type 5 (PDE-5) inhibitors. Barry H. Greenberg, MD, University of California, San Diego, La Jolla, California, USA, discussed clinical trial data for direct renin inhibitors and neprilysin inhibitors.
Several trials are ongoing or have been completed for aliskiren, which binds to the active site of renin to block production of angiotensin I. In the ALOFT trial [McMurray JJ et al. Circ Heart Fail 2008], aliskiren significantly reduced plasma brain natriuretic peptide (BNP) concentrations compared with placebo (p=0.0106) in patients with HF and a history of hypertension who had been treated with an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker and β-blocker.
Results were less promising in the ALTITUDE trial [Parving HH et al. N Engl J Med 2012] in which patients with type 2 diabetes who were at high risk for cardiovascular (CV) and renal events were randomized to receive aliskiren or placebo in addition to standard therapy. The trial was stopped prematurely due to a significantly higher occurrence of hyperkalemia (11.2% vs 7.2%) and hypotension (12.1% vs 8.3%) in the aliskiren group compared with the placebo group (p<0.001) and no apparent benefit.
Overall, aliskiren should be used with caution in this patient population due to the risk of hyperkalemia in patients on background renin-angiotensin-aldosterone system antagonists.
LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, significantly reduced N-terminal pro (BNP) compared with valsartan (p=0.005) in a Phase 2 trial in patients with HF [PARAMOUNT; Solomon SD et al. Lancet 2012]. The PARADIGM-HF study [NCT01035255], a Phase 3 trial comparing LCZ696 with enalapril in patients with HF with reduced ejection fraction, is currently underway.
Maya E. Guglin, MD, PhD, University of South Florida, Tampa, Florida, USA, presented data and guidelines for ivabradine, a selective If channel inhibitor. SHIFT [Swedberg K et al. Lancet 2010] enrolled 6558 patients with symptomatic HF, left ventricular ejection fraction (LVEF) of ≤35%, sinus rhythm with heart rate ≥70 bpm, on stable doses of background treatment, who had been admitted to the hospital for HF in the previous year.
The primary endpoint for SHIFT was the composite of CV death and hospital admission for worsening HF [Swedberg K et al. Lancet 2010]. In the ivabradine group, 24% of patients had a primary endpoint event compared with 29% of patients in the placebo group (p<0.0001). Based on these data, the European Society of Cardiology recommends the use of ivabradine in the situations outlined in Table 1 [McMurray JJ et al. Eur Heart J 2012].
European Society of Cardiology Recommendations for the Use of Ivabradine
Fady Malik, MD, PhD, Amgen, San Francisco, California, USA, provided an update on the status of omecamtiv mecarbil, a direct cardiac myosin activator. In the Phase 1, placebo-controlled, dose-escalation study, CY 1111 [Teerlink JR et al. Lancet 2011], omecamtiv mecarbil infusion resulted in dose-dependent increases in systolic ejection time (p<0.0001). Stroke volume, fractional shortening, and ejection fraction were also significantly increased at a dose of 0.5 mg/kg/hour of omecamtiv mecarbil (p<0.0001).
In the placebo-controlled, dose-ranging Phase 2 trial, CY 1121 [Cleland JG et al. Lancet 2011], omecamtiv mecarbil was given intravenously for 2, 24, or 72 hours to patients with HF and LV systolic dysfunction receiving guideline-indicated treatment. Placebo-corrected changes from baseline indicated concentration-dependent increases in LV ejection time and stroke volume (p<0.0001). A small reduction in heart rate was also noted (p<0.0001).
In both CY 1111 and CY 1121, there was no consistent pattern of adverse events in patients tolerant of all study-drug infusions. Myocardial ischemia occurred at high plasma concentrations of omecamtiv mecarbil and was the dose-limiting toxic effect.
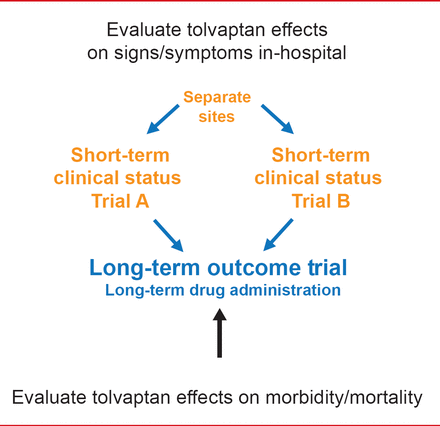
Marvin A. Konstam, MD, Tufts Medical Center, Boston, Massachusetts, USA, discussed the treatment of hyponatremia with vasopressin antagonists in patients with HF. EVEREST [Gheorghiade M et al. JAMA 2007; Konstam MA et al. JAMA 2007] was 2 identical short-term Phase 3 trials and one long-term outcome trial investigating the effects of tolvaptan, a vasopressin 2 receptor antagonist, in patients hospitalized for HF (Figure 1).
EVEREST Objectives
Reproduced with permission frm MA Konstam, MD.
The primary endpoints for EVEREST were all-cause mortality and CV death or hospitalization. No significant difference was observed in all-cause mortality (p=0.68), or the number of CV deaths or hospitalizations for HF (p=0.55) between the tolvaptan and placebo groups [Konstam MA et al. JAMA 2007].
However, in the EVEREST post hoc subgroup analysis of HF patients with hyponatremia (Na <130 mEq/L), the point estimate favored tolvaptan for all-cause mortality, and for the number of CV deaths or hospitalizations (p<0.05). According to Dr. Konstam, the data suggest that further studies are warranted with vaptans in HF patients who have hyponatremia.
Marc J. Semigran, MD, Harvard Medical School, Boston, Massachusetts, USA, provided an overview of PDE-5 inhibitor treatment in patients with HF. Several clinical trials have investigated the clinical viability of PDE5Is as adjunct treatment for HF.
The Chronic Sildenafil Treatment in Heart Failure trial [Guazzi M et al. Circ Heart Fail 2011] examined the effect of sildenafil in patients with stable systolic HF. Sildenafil significantly increased exercise capacity as measured by peak oxygen consumption (VO2) at 6 and 12 months of treatment compared with placebo (p<0.01). The ventilation relative to carbon dioxide production (VE/VCO2) slope was significantly decreased in the sildenafil group compared with the placebo group at 6 and 12 months (p<0.01).
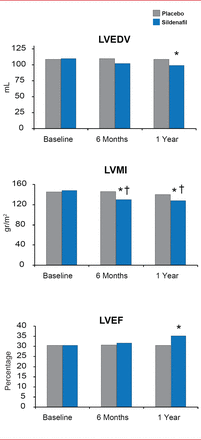
In the same study, sildenafil also improved LV systolic dysfunction by reversing LV remodeling. LV end-diastolic volume and LV mass index were significantly decreased in the sildenafil group compared with the placebo group (p<0.01). In addition, LVEF was significantly improved by sildenafil compared with placebo (p<0.01) [Guazzi M et al. Circ Heart Fail 2011]. The PDE5 Inhibition and Pulmonary Hypertension in Diastolic Heart Failure trial [Guazzi M et al. Circulation 2011] evaluated sildenafil treatment in patients with HF with preserved EF. By 6 months after the start of treatment, sildenafil had mediated significant improvements in right ventricular function (p<0.01) and pulmonary function (p<0.01). Pulmonary arteriolar resistance was decreased with sildenafil by 69%±18.0% (p<0.01).
Although further studies are still necessary to determine the role of most of these emerging agents in the treatment of HF, many may offer potential options for improving the future management of this challenging disease.
Chronic PDE5 Inhibition Improves Left Ventricular Systolic Dysfunction
LVEDV=left ventricular end diastolic volume; LVMI=left ventricular myocardial infarction; LVEF-left ventricular ejection fraction; PDE-5=phosphodiesterase type 5.
*p<0.01 vs placebo; #p<0.01 vs baseline.
Reproduced from Guazzi M et al. PDE5 Inhibition With Sildenafil Improves Left Ventricular Diastolic Function, Cardiac Geometry, and Clinical Status in Patients With Stable Systolic Heart Failure: Results of a 1-Year, Prospective, Randomized, Placebo-Controlled Study. Circulation Heart Failure 2011;4(1)8–17.
- © 2013 MD Conference Express®