Summary
In a trial focused on the treatment of stable angina in patients with type 2 diabetes, ranolazine significantly reduced the frequency of angina episodes compared with placebo. This article presents the results of the Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina study [TERISA; Kosiborod M et al. J Am Coll Cardiol 2013].
- Coronary Artery Disease
- Cardiology Clinical Trials
- Diabetes Mellitus
- Coronary Artery Disease
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Diabetes Mellitus
In a trial focused on the treatment of stable angina in patients with type 2 diabetes (T2DM), ranolazine significantly reduced the frequency of angina episodes compared with placebo. Mikhail Kosiborod, MD, St. Luke's Mid America Heart Institute, Kansas City, Missouri, USA, presented the results of the Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina study [TERISA; Kosiborod M et al. J Am Coll Cardiol 2013].
The primary objective of the randomized double-blind TERISA study was to evaluate the efficacy of ranolazine versus placebo on angina frequency in T2DM patients with coronary artery disease and chronic stable angina who were also taking 1 or 2 antianginal medications (eg, β-blockers). The primary endpoint was the average weekly number of angina episodes from Week 2 to Week 8 of treatment, while secondary endpoints included the average weekly number of sublingual nitroglycerin (SL NTG) doses from Week 2 to Week 8.
The trial enrolled 949 patients at 104 sites in Europe, Asia, and North America. Following a 4-week single-blind baseline-setting placebo period, patients (mean age 64 years) were randomized to receive ranolazine 1000 mg BID (n=473) or placebo (n=476) for 8 weeks. Eleven patients in each arm that either initiated or discontinued the study drug during the first 2 weeks were excluded from the final analysis. Researchers received daily data transmissions from patients who recorded angina episodes and SL NTG use in handheld electronic device diaries (98% compliance). Researchers followed-up with a phone call 2 weeks after the end of the 8-week period. Randomized patients were mostly male (61%) and had a mean diabetes duration of 7.5 years and a mean baseline HbA1C of 7.3%.
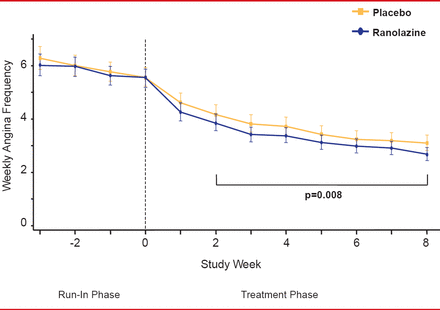
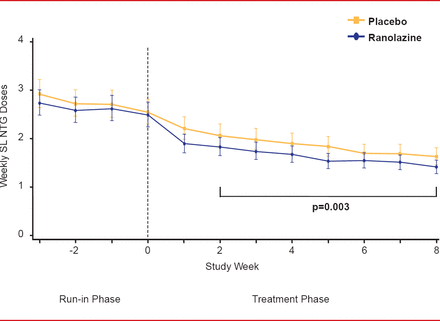
For the primary endpoint, patients in the ranolazine group experienced significantly fewer average weekly angina episodes from Week 2 to Week 8 than patients in the placebo group (3.8 vs 4.3,; p=0.008; Figure 1). Furthermore, patients in the ranolazine group took fewer average weekly SL NTG doses from Week 2 to Week 8 than those in the placebo group (1.7 vs 2.1, respectively; p=0.003; Figure 2). There were few serious adverse events, with no significant difference between the 2 groups.
Angina Frequency With Ranolazine Versus Placebo
Reproduced from Kosiborod M et al. Evaluation of Ranolazine in Patients with Type 2 Diabetes Mellitus and Chronic Stable Angina. Results from the TERISA randomized clinical trial. Journal of the American College of Cardiology Jan 2013; 10.1016/J.JACC.2013.02.011. With permission from Elsevier.
Average SL NTG Doses With Ranolazine Versus Placebo
SL NTG=sublingual nitroglycerin.
Reproduced from Kosiborod M et al. Evaluation of Ranolazine in Patients with Type 2 Diabetes Mellitus and Chronic Stable Angina. Results from the TERISA randomized clinical trial. Journal of the American College of Cardiology Jan 2013; 10.1016/J.JACC.2013.02.011. With permission from Elsevier.
It should be noted that generalizability of these results may be limited due to the lack of racial diversity of the study population. In addition, the short follow-up limits conclusions about the durability of therapy. Significant geographic heterogeneity was seen in treatment effect (p for interaction=0.016) with an apparent attenuation of benefit in selected Eastern European countries. Dr. Kosiborod said that an investigation is currently ongoing to determine the reason for this lack of an effect in these patients. In another subgroup analysis, the overall benefit with ranolazine versus placebo was more pronounced in patients with higher baseline HbA1C levels (p for interaction=0.027); however, measurement was not taken on follow-up for possible comparison.
In conclusion, TERISA showed that ranolazine was more effective than placebo in reducing angina frequency in T2DM patients with coronary artery disease and chronic stable angina. Future studies may shed light on potential dual effects of ranolazine on angina and glucose control in T2DM patients.
- © 2013 MD Conference Express®