Summary
Among statin-naïve patients with non-ST-segment elevation acute coronary syndrome managed with an early invasive strategy, pretreatment with high-dose rosuvastatin was associated with a significant reduction in the incidence of contrast-induced acute kidney injury. In addition, pretreatment with rosuvastatin was also associated with a reduction in adverse clinical events at 30 days compared with placebo. This article presents the results of the Protective Effect of Rosuvastatin and Antiplatelet Therapy on Contrast-Induced Acute Kidney Injury and Myocardial Damage in Patients With Acute Coronary Syndrome study [PRATO-ACS; NCT01185938].
- Lipid Disorders
- Renal Disease
- Cardiology Clinical Trials
- Lipid Disorders
- Renal Disease
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
Among statin-naïve patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) managed with an early invasive strategy, pretreatment with high-dose rosuvastatin was associated with a significant reduction in the incidence of contrast-induced acute kidney injury (CI-AKI). In addition, pretreatment with rosuvastatin was also associated with a reduction in adverse clinical events at 30 days compared with placebo. Anna Toso, MD, Misericordia e Dolce Hospital, Prato, Italy, presented the results of the Protective Effect of Rosuvastatin and Antiplatelet Therapy on Contrast-Induced Acute Kidney Injury and Myocardial Damage in Patients With Acute Coronary Syndrome study [PRATO-ACS; NCT01185938] on behalf of the trial investigators.
Statins, due to their lipid-lowering and pleiotropic properties, may have a renal-protective effect after contrast medium administration for patients undergoing an angiographic procedure. However, the dose, type, timing and target population for statin use is uncertain. This study tested the hypothesis that high doses of rosuvastatin given before an angiographic procedure would protect against the development of CI-AKI.
In the PRATO-ACS trial, statin-naïve NSTE-ACS patients admitted to the cardiac care unit (CCU) between July 2010 and August 2012 managed with an early invasive strategy were eligible for the study. Exclusion criteria were: emergent angiography, acute renal failure or early-stage renal disease requiring dialysis, a baseline serum creatinine ≥3 mg/dL, contraindications to statin treatment, or exposure to contrast medium within the last 10 days. After admission to the CCU, 271 patients were randomized to receive rosuvastatin (loading dose of 40 mg, then 20 mg/day) or a placebo. The primary endpoint was the development of CI-AKI defined as a rise in serum creatinine ≥0.5 mg/dL absolute or ≥25% increase relative to baseline that occurred within 72 hours of contrast exposure.
Serum creatinine increases ≥25%, ≥0.5 mg/dL, and ≥0.3 mg/dL within 48 and 72 hours, as well as a decrease in estimated glomerular filtration rate ≥25% within 72 hours were additional biomarker endpoints. Other clinical endpoints included acute renal failure requiring dialysis, persistent renal damage, all-cause mortality, myocardial infarction, and stroke through 30 days.
All patients were treated with dual antiplatelet therapy (aspirin+clopidogrel) prior to coronary angiography (±percutaneous coronary intervention) and after discharge. In addition, all patients received oral N-acetylcysteine and hydration both pre- and 24 hours post contrast medium (nonionic, dimeric iso-osmolar) administration. CI-AKI analysis was performed in 252 patients in each group after 72 hours. At discharge patients in the rosuvastatin pretreatment group continued rosuvastatin 20 mg, while those in control group received atorvastatin 40 mg daily.
There were no significant differences in baseline clinical, biochemical, or demographic characteristic, time from randomization to angiography, procedural success, or CI-AKI Mehran risk score, a validated risk prediction model for the development of CI-AKI, between the 2 groups.
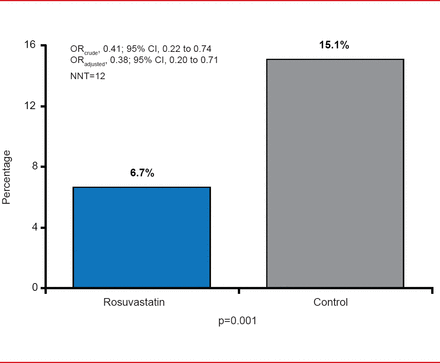
CI-AKI was significantly less frequent in patients pretreated with rosuvastatin compared with placebo (6.7% vs 15.1%; adjusted OR, 0.38; 95% CI, 0.20 to 0.71; p=0.001; Figure 1). Compared with placebo, pretreatment with rosuvastatin was associated with significant reductions in all of the CI-AKI endpoints and the effect was consistent across all prespecified subgroups.
Primary Endpoint: CI-AKI
CK-AKI=contrast-induced acute kidney injury; NNT=number needed to treat.
Reproduced with permission from A Toso, MD.
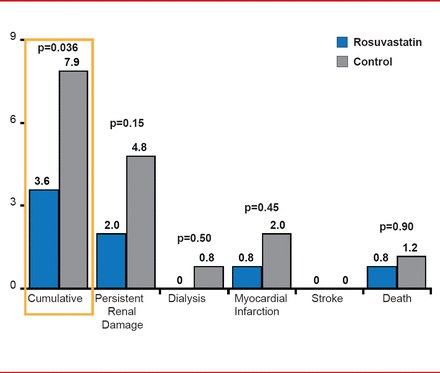
In addition, rosuvastatin pretreatment reduced the rate of acute renal failure requiring dialysis, persistent renal damage, all-cause mortality, myocardial infarction, and stroke at 30 days (3.6% vs 7.9%; p=0.036) compared with placebo (Figure 2).
Adverse Clinical Events (30 Days)
Reproduced with permission from A Toso, MD.
To date, there have been few effective strategies to protect patients from developing CI-AKI. The findings from the PRATO-ACS trial are notable and further studies are needed to both corroborate these results and potentially evaluate whether this is a class-effect of statins or unique to rosuvastatin.
- © 2013 MD Conference Express®