Summary
The results of this large randomized controlled trial in patients with well-controlled lipid levels but who are at a high risk of cardiovascular events once again call into question the the clinical benefits of niacin despite an increase in high-density lipoprotein cholesterol (HDL-C), and reductions in triglyceride levels and low density lipoprotein cholesterol levels. This article presents the results of the Treatment of HDL to Reduce the Incidence of Vascular Events trial [HPS2-THRIVE Collaborative Group. Eur Heart J 2013].
- Cardiology Clinical Trials
- Lipid Disorders
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
- Lipid Disorders
The results of this large randomized controlled trial in patients with well-controlled lipid levels but who are at a high risk of cardiovascular events once again call into question the the clinical benefits of niacin despite an increase in high-density lipoprotein cholesterol (HDL-C), and reductions in triglyceride levels and low density lipoprotein cholesterol (LDL-C) levels. Jane M. Armitage, MD, Oxford University, Oxford, United Kingdom, presented the results of the Treatment of HDL to Reduce the Incidence of Vascular Events trial [HPS2-THRIVE Collaborative Group. Eur Heart J 2013]. Prof. Armitage reported that adding extended-release niacin plus laropiprant (ERN/LRPT), an antiflushing agent, to background therapy with simvastatin (with or without ezetimibe) did not reduce the risk of heart attack, stroke, and revascularizations, and was associated with significantly increased serious adverse events (SAEs).
HPS2-THRIVE randomized 25,673 patients from China, United Kingdom, and Scandinavia with a prior history of myocardial infarction (MI), ischemic stroke or transient ischemic attack, peripheral arterial disease, or diabetes with other cardiovascular disease, on a background of simvastatin with or without ezetimibe, to either ERN 2 g plus LRPT 40 mg daily (n=12,838) or placebo (12,835). Lipid stabilization was achieved prerandomization using simvastatin 40 mg daily (with or without ezetimibe) to target total cholesterol of 135 mg/dL and patients were followed for 4 years. The primary endpoint was time to first major vascular event, defined as a nonfatal MI or coronary death, nonfatal or fatal stroke, or revascularization. Mean baseline total cholesterol, direct-LDL, HDL, and triglycerides levels were 128, 63, 44, and 125 mg/dL, respectively, which were below the targets for such high-risk patients.
By the end of the 4-year study, 25% of the ERN/LRPT and 17% of the placebo-treated patients had discontinued study treatment and the average overall compliance with ERN/LRPT was 78%. SAEs, including new-onset diabetes, occurred significantly more often in the ERN/LRPT group versus placebo (Table 1). In addition, myopathy was more common in the ERN/LPRT arm (0.6%) versus placebo (0.1%; 95% CI, 2.62 to 7.50; p<0.0001) and much higher in China than in Europe (0.13%/year vs 0.04%/year; p=0.001).
SAEs With ERN/LRPT
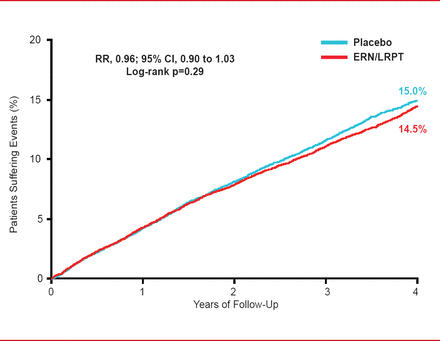
On average, treatment with ERN/LRPT decreased LDL-C by 10 mg/dL and triglycerides by 33 mg/dL, while increasing HDL-C by 6 mg/dL. There was no significant reduction in the primary endpoint (major coronary event, stroke, or revascularization) with ERN/LRPT compared with placebo after 4 years (14.5% vs 15.0%, RR, 0.96; 95% CI, 0.90 to 1.03; p=0.29; Figure 1).
There was an overall significant reduction in revascularizations with ERN/LRPT versus placebo (revascularization events, 6.3% vs 7.0%; p=0.03) with no significant differences in any of the other components of the primary endpoint, including coronary death, nonfatal MI, ischemic stroke, or hemorrhagic stroke. All-cause death tended to occur more frequently with ERN/LRPT than placebo (6.2% vs 5.7%; RR, 1.09; 95% CI, 0.99 to 1.21; p=0.08).
Effect of ERN/LRPT on Major Vascular Events
ERN/LRPT=extended-release niacin plus laropiprant.
Reproduced with permission from J Armitage, MD.
There were no significant differences in any of the subgroup comparisons based on age, sex, baseline disease category, or type of statin-based therapy, except for an excess of statin-related myopathy in Chinese patients.
The findings of HPS-THRIVE are consistent with those of previous niacin trials, including AIM-HIGH [AIM-HIGH Investigators. N Engl J Med 2011], and suggest lack of clinical benefit with niacin in the treatment and prevention of cardiovascular disease among patients receiving statin therapy. Whether other therapies (eg, CETP inhibitors, PCSK9 inhibitors) that increase HDL-C and/or lower LDL-C may be beneficial in addition to statins are being explored in ongoing Phase 3 trials.
- © 2013 MD Conference Express®