Summary
At this year's International Stroke Conference, leaders in the field of stroke met to review some of the important updates to stroke-related guidelines. Included are some of the important revisions to the Guidelines for the Early Management of Patients With Acute Ischemic Stroke [Jauch EC et al. Stroke 2013]; 2012 Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage; an overview and recommendations for the use of the warfarin, dabigatran, apixaban, and rivaroxaban; and a discussion of the relationship between stroke and cardiovascular disease.
- Ischemia
- Thrombotic Disorders
- Arrhythmias Guidelines
- Prevention & Screening
- Neurology
- Ischemia
- Thrombotic Disorders
- Arrhythmias
- Neurology Guidelines
- Prevention & Screening
At this year's International Stroke Conference, leaders in the field of stroke met to review some of the important updates to stroke-related guidelines.
Edward C. Jauch, MD, MS, Medical University of South Carolina, Charleston, South Carolina, USA, discussed some of the important revisions to the Guidelines for the Early Management of Patients With Acute Ischemic Stroke [Jauch EC et al. Stroke 2013].
Key areas of focus include the importance of stroke systems of care, streamlining processes to minimize time, the importance of reperfusion (intravenous [IV] and intra-arterial), and expanded eligibility for reperfusion. The revised guidelines state that “patients should be transported rapidly to the closest available certified primary stroke center or comprehensive stroke center…” and they stress the importance of emergency medical services in prehospital notification. There are revised guidelines on emergency evaluation and diagnosis of stroke, particularly with respect to testing prior to initiation of IV tissue plasminogen activator (tPA) and the use optimal use of imaging. Revised recommendations for IV fibrinolysis now call for a door-to-needle time that is within 60 minutes from hospital arrival. There are several revised recommendations concerning eligibility criteria for the use of tPA, and there are new recommendations addressing endovascular interventions.
Alejandro Rabinstein, MD, Mayo Clinic, Rochester, Minnesota, USA, reviewed the highlights from the 2012 Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage (aSAH) [Connolly ES et al. Stroke 2012] in the following areas:
Risk Factors and Prevention
-
▪ Improved guidance for the treatment of hypertension (HTN).
Natural History and Outcome
-
▪ Determine severity early using Hunt-Hess or World Federation Neurosurgeons Scale.
-
▪ Evaluate and treat suspected aSAH as soon as possible to reduce the risk of rebleeding.
-
▪ Introduce multidisciplinary and comprehensive follow-up.
Diagnosis and Clinical Presentation
-
▪ Computed tomography angiography may be considered, but, if inconclusive, use digital subtraction angiography (DSA).
-
▪ Magnetic resonance imaging can be considered for emergency diagnosis if CT is nondiagnostic, but it does not obviate the need for a lumbar puncture.
-
▪ DSA with 3D rotational images is indicated before deciding on a treatment approach.
Medical Measures to Prevent Rebleeding
-
▪ Control HTN with a titratable agent (decreasing systolic blood pressure to <160 mm Hg is reasonable).
-
▪ If treatment is delayed and there are no compelling contraindications, short-term (<72 hours) treatment with tranexamic acid or aminocaprioic acid is reasonable.
-
▪ Coiling should be considered for aneurysms that are amenable to both coiling and clipping.
Vasospasm
-
▪ Oral nimodipine is recommended.
-
▪ Maintain euvolemia and normal circulating volume to prevent delayed cerebral ischemia (DCI).
-
▪ Perfusion imaging can be useful.
-
▪ Induced HTN is recommended when DCI occurs unless precluded by cardiac status.
Seizures
-
▪ Prophylactic antiepileptic drugs (AEDs) in the immediate posthemorrhagic period may be considered.
-
▪ Routine use of AEDs is not recommended but may be considered for patients with risk of delayed seizure disorder.
Medical Complications
-
▪ Avoid hypotonic fluids and intravascular volume contraction.
-
▪ Monitor volume status in certain patients by central venous pressure and/or pulmonary wedge and/or fluid balance and treat volume contraction with crystalloids or colloids.
-
▪ Maintain normothermia.
-
▪ Consider careful glucose management with strict avoidance of hypoglycemia.
-
▪ Red blood cell transfusions to treat anemia may be reasonable (optimal hemoglobin goal is unknown).
-
▪ Fludrocortisone and hypertonic saline are reasonable to prevent and treat hyponatremia.
-
▪ Early identification and targeted treatment of heparin-induced thrombocytopenia and deep vein thrombosis are recommended.
Dr. Rabinstein said that most cases of aSAH are eminently treatable and the objective should be full functional recovery.
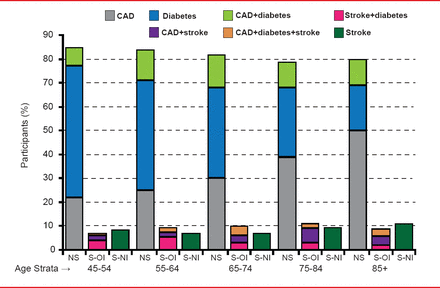
Daniel T. Lackland, DrPH, Medical University of South Carolina, Charleston, South Carolina, USA, provided evidence and recommendations for the inclusion of patients with stroke among those considered to be at high risk of cardiovascular disease (CVD) as well as part of the outcome cluster in risk prediction instruments for vascular disease [Lackland DT et al. Stroke 2012]. The evidence for the inclusion of stroke in the vascular outcome cluster is that many of the same risk factors and mechanisms that cause heart disease also cause stroke, and many treatments (antihypertensive treatment, statins) that reduce the risk of heart disease also reduce the risk of stroke. The inclusion of stroke as an outcome could lead to an increase in the absolute risks of vascular events by 5% to 10% and thus, detect additional patients eligible for more intensive preventive interventions. Inclusion of atherosclerotic stroke among the categories of risk equivalents is shown in Figure 1.
Stroke as a Risk Equivalent: Percentage of Participants With Levels of Risk by Age
CAD=coronary artery disease; NS=no stroke; S-OI=stroke otherwise identified; S-NI=stroke newly identified.
Reproduced from Lackland DT et al. Inclusion of Stroke in Cardiovascular Risk Prediction Instruments: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke Jul 2012;43(7):1998–2027. With permission from Lipincott Williams and Wilkins.
Dr. Lackland suggests the following regarding stroke and risk assessment:
-
Large vessel atherosclerotic ischemic stroke should be considered as coronary heart disease risk equivalent.
-
Ischemic stroke can reasonably be considered a relevant outcome along with coronary heart disease (CHD) outcomes in CVD risk prediction instruments.
-
Ischemic stroke subtypes other than large vessel atherosclerosis may reasonably be considered as CHD risk equivalents; future research is needed.
-
Hemorrhagic strokes and strokes of undetermined subtypes may be included among outcomes in general CVD risk prediction instruments.
-
Ischemic stroke can reasonably be considered a relevant outcome in clinical 10-year cardiovascular risk prediction instruments.
-
Further clinical epidemiological studies are needed.
In a discussion of the current optimal therapy for stroke prevention in atrial fibrillation (AF), Karen L. Furie, MD, Brown University, Providence, Rhode Island, USA, provided an overview and recommendations for the use of the warfarin, dabigatran, apixaban, and rivaroxaban.
Evidence for the use of dabigatran, an oral pro-drug and competitive inhibitor of Factor II, comes from the RE-LY study [Connolly SJ et al. N Engl J Med 2009], which showed that dabigatran 110 mg was similar to warfarin for stroke prevention, with lower rates of major hemorrhage, and 150 mg was superior to warfarin but had similar rates of major hemorrhage.
Rivaroxaban is a direct factor Xa inhibitor that is noninferior to warfarin for the prevention of stroke, systemic embolism, or the risk of major bleeding. This was confirmed in both the ROCKET AF study [Patel MR et al. N Engl J Med 2011] and the J-ROCKET study that also confirmed the safety and efficacy of rivaroxaban in patients with moderate renal impairment and preserved renal function [Hori M et al. Circ J 2012].
In the AVERROES trial, apixaban (another factor Xa inhibitor) reduced the risk of stroke and systemic embolism relative to aspirin without significantly increasing the risk of major bleeding or intracranial hemorrhage in patients with AF for whom vitamin K antagonist therapy was unsuitable [Connolly SJ et al. N Engl J Med 2011]. Apixaban was also shown to be superior to warfarin for preventing hemorrhagic stroke and similar for preventing ischemic stroke in the ARISTOTLE trial. It caused less bleeding and lowered mortality [Granger CB et al. N Engl J Med 2011].
A comparison overview of these new agents versus warfarin is shown in Table 1.
Overview of New Agents Versus Warfarin
The use of warfarin, dabigatran, apixaban, and rivaroxaban are all indicated for stroke prevention (Class I-IIa, Level of Evidence A-B). Although the safety and efficacy of combining dabigatran, rivaroxaban, or apixaban with an antiplatelet agent has been established at a Class IIb, Level of Evidence C, Dr. Furie feels that the use of these agents in combination with antiplatelet therapy should be considered with caution. When selecting optimal therapy for stroke prevention in AF, vascular neurologists should base their decision on risk factors, cost, tolerability, patient preference, drug interactions, and compliance. Patients should be educated about the need for careful transitions and potential risks.
The editors would like to thank the many members of the American Stroke Association presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2013 MD Conference Express®