Summary
Stroke is the second leading cause of death with 16 million strokes occurring worldwide each year. The randomized Interventional Management of Stroke III Trial [IMS III] study was designed to test whether endovascular therapy following IV tPA was more efficacious than IV tissue plasminogen activator (tPA) alone [Broderick JP et al. N Engl J Med 2013].
- Interventional Techniques & Devices
- Neurology
- Interventional Techniques & Devices
- Exclusive Article - For home page
Joseph P. Broderick, MD, University of Cincinnati, Cincinnati, Ohio, USA, presented data from the randomized Interventional Management of Stroke III Trial [IMS III] study designed to test whether endovascular therapy following IV tPA was more efficacious than IV tissue plasminogen activator (tPA) alone [Broderick JP et al. N Engl J Med 2013].
IMS III was planned to enroll 900 subjects aged 18 to 82 years with an National Institutes of Health Stroke Scale (NIHSS) ≥10, or an NIHSS 8 to 9 with computed tomography angiography (CTA) evidence of internal carotid artery (ICA), M1, or basilar occlusion, prior to administration of IV tPA. Subjects were randomized 2:1 to endovascular therapy following IV tPA (n=434) or standard IV tPA alone (n=222). In the endovascular therapy group, tPA (∼0.6 mg/kg) was to be administered within 3 hours of stroke onset, followed by angiography to determine if a treatable thrombus was present. If present, endovascular therapy proceeded using intra-arterial of administration of tPA at the site of the vessel occlusion or a thrombectomy device cleared for use by the FDA. The endovascular treatment began within 5 hours and had to be completed within 7 hours of stroke onset. The control arm received standard full dose tPA (0.9 mg/kg over one hour). The primary outcome was a modified Rankin Scale (mRS) score of 0 to 2, or functional independence at 3 months. The primary safety measure was mortality at 3 months and symptomatic intracerebral hemorrhage (ICH) within 30 hours of randomization.
IMS III was stopped for futility after enrolling 656 patients; there were no significant safety concerns. Time from onset to start of endovascular therapy was 249 minutes. There was no significant difference in the primary outcome between treatment arms even when accounting for onset to IV tPA, Alberta Stroke Program Early CT score, or a positive pretreatment CTA showing occlusion of the internal carotid artery, middle cerebral artery trunk, or basilar artery. There was a trend toward significance (p=0.06) in patients with a baseline NIHSS ≥20 favoring endovascular treatment. Within 90 days, 19.1% of the patients in the endovascular group died versus 21.6% in the IV tPA group. Asymptomatic ICH (p=0.01) and subarachnoid hemorrhage (p=0.02) within 30 hours of IV tPA initiation were significantly higher in the endovascular group.
Andrew M. Demchuk, MD, Hotchkiss Brain Institute, University of Calgary, Calgary, Alberta, Canada, discussed the impact of CT and magnetic resonance angiography (MRA) in the IMS III trial based on location of occlusion. Of the 656 subjects in the trial, 292 had baseline CTAs and 14 had baseline MRAs (total 47%). Results presented at the conference were limited to ICA, M1, M2, and basilar segments of the IA circulation. Grade 1 (complete occlusion) and Grade 2 (hairline lumen) were considered occlusion of that segment.
In the endovascular arm, recanalization was achieved in 85.71% of subjects with 24 hour CTA versus 60.87% in the IV tPA-only (p<0.0001) group. The 24-hour recanalization rates were high in both arms for middle cerebral artery occlusions. The 90-day clinical outcomes with mRS 0 to 2 for isolated M1 occlusions were similar. Low 24-hour recanalization rates were seen in IV tPA arm for ICA occlusions. A post hoc analysis of carotid T/L occlusions and tandem ICA plus M1 showed greater recanalization and better outcomes with combined (IV tPA + endovascular) treatment compared with IV tPA alone. Future endovascular/thrombolytic trial design should include baseline vascular imaging given the wide differences in clinical effects by occlusion site seen in IMS III.
Stroke is becoming a global burden in the developing world, which has little access to modern endovascular treatment resources. Stephen M. Davis, MD, Royal Melbourne Hospital, University of Melbourne, Victoria, Australia, outlined the challenges many low-income regions face regarding stroke treatment.
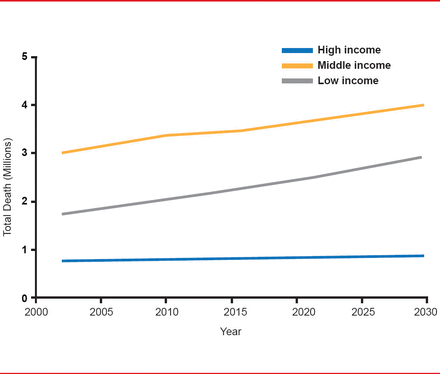
Stroke is the second leading cause of death with 16 million strokes occurring worldwide each year. Between 1990 and 2010, stroke increased by 26% worldwide—mostly in developing countries [Lozano R et al. Lancet 2012]. At the same time, there has been more than a 100% increase in low- to middle-income countries, there has been a 42% decrease in high-income countries (Figure 1) [Feigin VL et al. Lancet Neurol 2009].
Although there have been major changes in stroke management in high-income and some rapidly developing economies such as the use of stroke units, tPA, telemedicine, endovascular clot retrieval, and the use of comprehensive stroke centers, these are not widespread and even basic stroke services are often lacking in poorer regions. Higher rates of death and disability from stroke in these regions are due a lack of primary care treatment to screen patients for and mitigate risk factors; the lack of access to common drugs and basic medical equipment; the lack of poststroke follow-up programs and rehabilitation, and secondary stroke prevention [Norriving B, Kissela B. Neurology 2013].
Projected Trends for Stroke Deaths by World Bank Income Group: 2002–2030
Reproduced from Feigin VL et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurology 2009;8(4):355–369. With permission from Elsevier.
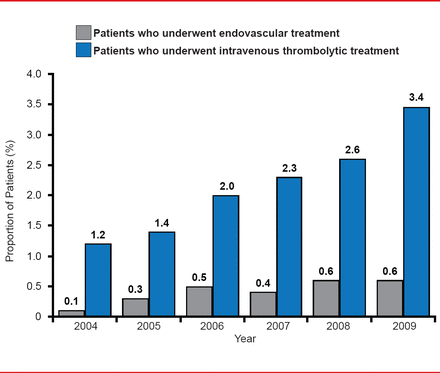
Increased use of tPA holds great potential in the developing world. For example, Pakistan with a population of 160 million has only 5 stroke units and 2 private hospitals where tPA is available. In China, there are 3.76 million strokes annually but only about 1.3% of these patients receive tPA. Extracranial and intracranial stenting is common, but only around 0.05% of eligible patients receive clot retrieval outside of first world countries and those with rapidly developing economies such as China, India, Brazil, and Russia. Although United States national trends for the use of IV tPA and thrombectomy are increasing (Figure 2), in most other countries they are underutilized.
Stroke units are effective for assessment and monitoring, acute management, and discharge planning [Langhorne P et al. Lancet Neurol 2012], and should be a first priority for the majority of regions.
Keith Muir, MD, University of Glasgow, Glasgow, United Kingdom, suggested some reasons the IMS III study may have reached the futility cutoff, including the use of older thrombectomy devices, long onset to treatment times, overoptimistic effect size estimate, and inadequate patient selection.
US National Trends in Mechanical Thrombectomy and IV tPA
Reproduced from Hassan AE et al. National Trends in Utilization and Outcomes of Endovascular Treatment of Acute Ischemic Stroke Patients in the Mechanical Thrombectomy Era. Stroke 2012;43(11):3012–3017. With permission from Lipincott Williams and Wilkins.
Mechanical thrombectomy after IV tPA seems as safe as mechanical thrombectomy alone [Smith WS. Am J Neuroradiol 2006]. Endovascular therapy may be advantageous in other settings including primary therapy <4.5 hours for patients not eligible for IV tPA, as primary therapy <4.5 hours instead of IV tPA, and as rescue therapy for IV tPA nonresponders. What is striking about the thrombectomy studies is the lack of correlation between high rate of recanalization achieved and the clinical outcome. Mortality and morbidity increase with time, and late recanalization can lead to poor clinical outcomes. Although adding imaging to the schedule is useful, it also adds time to the recanalization procedure, and thus comes with a built-in penalty. Odds of a complete recovery (mRS 0 to 1) decline rapidly from 1.5 hours to 4.5 to 5 hours [Lees KR et al. Lancet 2010]. This information needs consideration when designing new trials. There are many ongoing trials with strategies that are varied and overlapping. Most use noninvasive angiographic selection by CTA, MRA, or very thin slice noncontrast CT. More rapid treatment with endovascular therapy, better patient selection criteria, appropriate clinical scores, good angiographic data, and evidence of tissue viability will likely be important to improve the likelihood for favorable outcome with endovascular therapy. Effect size and sample size must be considered carefully.
- © 2013 MD Conference Express®