Summary
This article presents 180-day data from the Intraoperative CT-Guided Endoscopic Surgery trial [ICES] showing that early CT-guided endoscopic surgery is safe and associated with improved neurological outcomes compared with medical management in patients with primary intracerebral hemorrhage. ICES is a substudy of the Minimally Invasive Surgery Plus rtPA for Intracerebral Hemorrhage Evacuation study [MISTIE; NCT00224770].
- Ischemia
- Neuroimaging Clinical Trials
- Interventional Techniques & Devices
- Ischemia
- Neuroimaging
- Neurology Clinical Trials
- Interventional Techniques & Devices
- Neurology
Paul Vespa, MD, University of California, Los Angeles, Los Angeles, California, USA, presented 180-day data from the Intraoperative CT-Guided Endoscopic Surgery trial [ICES] showing that early CT-guided endoscopic surgery is safe and associated with improved neurological outcomes compared with medical management in patients with primary intracerebral hemorrhage (ICH).
ICES is a substudy of the Minimally Invasive Surgery Plus rtPA for Intracerebral Hemorrhage Evacuation study [MISTIE; NCT00224770]. The inclusion and exclusion criteria for ICES were the same as for MISTIE to facilitate a prespecified comparison between ICES surgery and the combined ICES plus MISTIE medical control arm. Randomization and surgery took place within 48 hours of stroke onset. Two serial CT scans were performed ≥6 hours apart to ensure that the hematoma remained stable. The ICH volume threshold was >20 cc; some intraventricular hemorrhage was permitted.
Surgery was accomplished using a stereotactic navigational scan. The endoscope was inserted two thirds of the way into the hematoma along its long axis, and suction and then irrigation were applied for variable amounts of time. The endoscope was backed off to about one third of the depth, and suction and irrigation were repeated before the instrument was withdrawn. A postoperative CT scan was performed. The primary study endpoint was safety. Secondary endpoints included volume reduction, surgical serious adverse events, and modified Rankin Scale (mRS) score at 180 and 365 days.
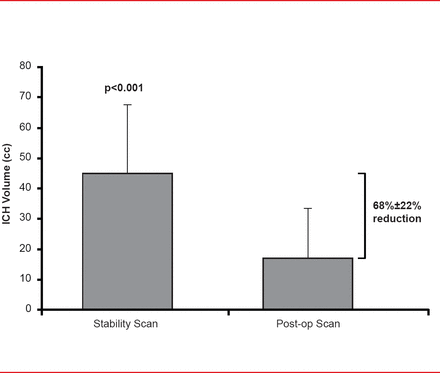
Subjects (mean age, ∼62 years; mostly men) were randomly assigned to endoscopic surgery (n=18) or medical management (n=6). Mean time to surgery was 32.8±14 hours. Endoscopic surgery resulted in a 68%±22% immediate volume reduction (p<0.001; Figure 1) with the volume being reduced to <15 cc in 67% of patients. Volume was unchanged in the medically managed patients after 72 hours. Mortality at 7, 30, and 180 days post onset was significantly higher in the medical (0%, 7%, and 60%, respectively) versus the surgical arm (0%, 4%, and 13%, respectively; p<0.01). One surgical patient had immediate nonfatal postoperative rebleeding. There was no immediate surgical-related mortality.
Volume Reduction
Reproduced with permission from P Vespa, MD.
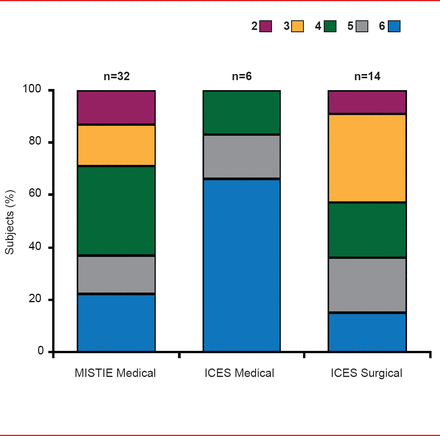
At 180 days, good neurological outcome (mRS score 0 to 3) was more frequent in patients receiving surgery (45%) compared with those on medical therapy (0%; Figure 2). On a prespecified intention-to-treat secondary analysis of ICES surgery versus combined medical controls in ICES plus MISTIE (n=13 vs n=36) at 180 days, the proportion of mRS scores 0 to 3 remained 15% greater in ICES surgery versus combined medical controls (38% vs 23%) [Vespa P et al. ISC 2013 (abstr LB2)].
Functional Outcomes: mRS at Day 180
Reproduced with permission from P Vespa, MD.
Dr. Vespa noted that endoscopic surgery for ICH is safe (eg, lower mortality at all time points compared with medical treatment), results in an immediate 70% reduction in ICH volume, and conveys a 15% advantage for a good clinical outcome (mRS score ≤3) after 180 days. Despite some variability in surgical technique—particularly with respect to suction, the procedure is generalizable and reproducible across multiple centers and surgeons.
- © 2013 MD Conference Express®