Summary
Cryptogenic stroke remains a major challenge for clinicians taking care of patients who have had strokes. Patent foramen ovale (PFO) is a contributor to cryptogenic stroke due to paradoxical embolism [Furlan AJ et al. N Engl J Med 2012], but the optimal management strategy for PFO has yet to be defined [Kitsios GD et al. Stroke 2012]. This article reports the results of a follow-up analysis of the RESPECT PFO Clinical Trial [RESPECT; NCT00465270] to characterize the qualifying and endpoint ischemic strokes.
- Cerebrovascular Disease Clinical Trials
- Neurology
- Cerebrovascular Disease
- Neurology Clinical Trials
Cryptogenic stroke remains a major challenge for clinicians taking care of patients who have had strokes. Patent foramen ovale (PFO) is a contributor to cryptogenic stroke due to paradoxical embolism [Furlan AJ et al. N Engl J Med 2012], but the optimal management strategy for PFO has yet to be defined [Kitsios GD et al. Stroke 2012]. Jeffrey L. Saver, MD, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California, USA, reported the results of a follow-up analysis of the RESPECT PFO Clinical Trial [RESPECT; NCT00465270] to characterize the qualifying and endpoint ischemic strokes.
RESPECT included patients aged 18 to 60 with PFO who had a cryptogenic stroke within 270 days. Enrollment continued until the 25th endpoint. Patients were randomized to the device group (n=499) or the medical group (n=481). The device group received closure with the AMPLATZER PFO Occluder plus medical therapy, and the medical group was scheduled to receive 1 of 5 medical treatment regimens (aspirin, warfarin, clopidogrel, or aspirin with dipyridamole); however the fifth treatment regimen of aspirin with clopidogrel was removed from the protocol as it was no longer included in the American Heart Association/American Stroke Association treatment guidelines.
Patients were excluded from the trial if they had cerebral, cardiovascular, and/or systemic conditions that suggested mechanisms other than PFO were responsible for the stroke. These mechanisms included atrial fibrillation, carotid disease, cardiomyopathy, small artery disease, uncontrolled diabetes mellitus or hypertension, arterial hypercoagulable states, or other sources of right-to-left shunt.
Primary analysis of the trial has previously shown that there was a 46.6% to 72.7% reduction in risk of stroke in the device versus the medical therapy group [Carrol JD. TCT 2012]. In the follow-up analysis, the basic features of the stroke patients were similar in the device and medical groups. The topography and lesion size of the qualifying strokes were also well balanced between the 2 groups. There was a trend towards magnified value of the device compared with the medical group in patients whose qualifying events occurred in the setting of atrial septal aneurysms (p=0.016), a large shunt size (p=0.012), or in an isolated superficial distribution (p=0.049).
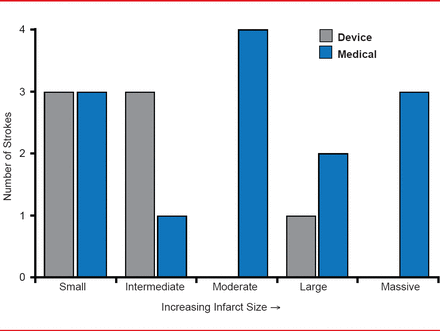
The topography of endpoint ischemic strokes was significantly different between the medical and device groups. The medical group had a larger proportion of strokes that were superficial, mixed superficial and deep, or otherwise not small and deep in distribution compared with the device group (p=0.04). In addition, the medical group experienced more infarcts ≥1.5 cm (69.2%) than the device group (14.3%; p=0.06). Figure 1 shows a general shift towards larger lesion size in the medical group compared with the device group.
Lesion Size of Endpoint Ischemic Strokes
Reproduced with permission from JL Saver, MD.
Limitations of the RESPECT trial include the limited power of the subgroup interaction analysis with only 25 events to explore. In addition, the work-up of endpoint events was incomplete in some cases since some strokes were evaluated at nonstudy centers, and all patients had already had a complete evaluation for qualifying infarcts, so not all tests were repeated in every patient.
Dr. Saver said, “Consideration of the neurovascular aspects of the RESPECT trial reinforce the primary analysis.” When patients were stringently selected to identify those with a history of cryptogenic stroke and PFO, closure with the AMPLATZER PFO Occluder showed evidence of benefit over medical management alone. The device was more effective at averting infarcts associated with a paradoxical embolic stroke mechanism, including those with superficial vascular distribution, convexity strokes, and strokes of larger size, providing additional evidence of a biological effect of closure with the AMPLATZER PFO Occluder.
- © 2013 MD Conference Express®