Summary
This article presents results from the Minimally Invasive Surgery Plus tPA for Intracerebral Hemorrhage Evacuation trial [MISTIE; NCT00224770], which showed that catheter-based clot reduction plus tissue plasminogen activator (MIS + tPA) is safe and may reduce long-term disability after intracerebral hemorrhage.
- Neurology Clinical Trials
- Interventional Techniques & Devices
- Ischemia
- Neurology Clinical Trials
- Neurology
- Interventional Techniques & Devices
- Ischemia
Daniel Hanley, MD, Johns Hopkins University, Baltimore, Maryland, USA, presented results from the Minimally Invasive Surgery Plus tPA for Intracerebral Hemorrhage Evacuation trial [MISTIE; NCT00224770], which showed that catheter-based clot reduction plus tissue plasminogen activator (MIS + tPA) is safe and may reduce long-term disability after intracerebral hemorrhage.
Volume of intracerebral hemorrhage (ICH) is the strongest predictor of 30-day outcome for all locations of spontaneous ICH [Broderick JP et al. Stroke 1993]. MISTIE was a 2-stage multicenter, Phase 2 trial that examined outcomes and cost benefit of reducing clot size by using a catheter inserted into the largest part of the clot to apply tPA every 8 hours for 3 days. The study included patients with spontaneous supratentorial ICH ≥20 cc (stable ≥6 hours post diagnosis as shown on computed tomography) who were treated with either MIS plus tPA (n=54) or standard medical care (n=42) and followed for 180 days for stage I and 365 days for stage II. Participants were mean age 61 years (55.2% white, 65.6% men). Most were hypertensive (86.5%) and 26.5% had a diagnosis of diabetes. Prior smokers were more common in the surgical group (31.5%) versus those receiving standard medical therapy (7.1%). Of the subjects, 75% received their surgery between 12 and 38 hours post event.
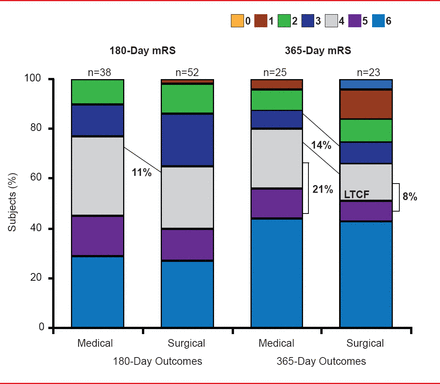
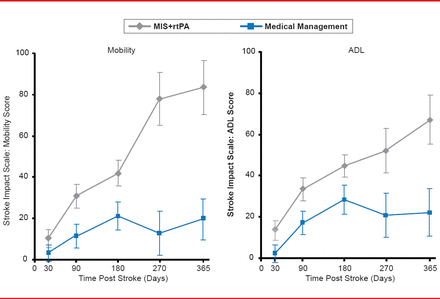
At 365 days there was a 14% difference in functional performance (defined as 0 to 3 vs 4 to 6 on the modified Rankin Scale [mRS]) favoring MIS plus tPA (slightly greater than the 11% difference observed at 180 days). The improvement included a differential shift to higher independence levels at mRS 0 to 2 (Figure 1). Similar magnitudes of improvement (Figure 2) were also seen when the subjects were evaluated for improvements in mobility and activities of daily living using the Stroke Impact Scale [Duncan PW et al. Stroke 1999].
Functional Outcomes
LTCF=long-term care facility; mRS=modified Rankin Scale.
Reproduced with permission from D Hanley, MD.
Functional Improvements: Stroke Impact Scale
ADL=activities of daily living; MIS=minimally invasive surgery; rtPA=recombinant tissue plasminogen activator.
Reproduced with permission from D Hanley, MD.
The improvements were also reflected in the cost of care. Although the median length of intensive care unit (ICU) stay was similar for both groups (9 days for patients in the surgical group vs 8 days for patients in the standard medical therapy group) the median hospital stay was 38 days shorter for the MIS plus tPA group (p=0.015). In addition, fewer surgical patients were residing in long-term care facilities both at 180 (17% vs 24%; not significant) and 365 days (8% vs 21%; not significant). After accounting for all costs (eg, ICU stay, MIS + tPA procedures), the results indicate that the experimental procedure saves an estimated $44,329 of medical care costs per patient [Hanley DF et al. ISC 2013 (abstr LB1)].
Similar results were obtained for all subgroups. Importantly, the treatment effect does not seem to be mitigated by the depth or size of the hematoma or the time to surgery. There is a progressive improvement in mRS outcomes as the amount of clot removed is increased.
Dr. Hanley believes that reduction in clot burden is a mechanism of benefit, MIS plus tPA saves tissue at risk, and secondary injury occurs over days—not just immediately. “Most likely MIS plus tPA increases independence and appears to improve function and decrease cost,” he said. This procedure needs to be tested in a large Phase 3 trial.
- © 2013 MD Conference Express®