Summary
During an invited symposium at the 2013 International Stroke Conference, investigators shared their thoughts on key areas in stroke research that will shape the field in a number of ways. Topics include: an epidemiological look at stroke mortality and its global health burden, a review of the current state of clinical trial research, a discussion of the need for the recognition of and attention to vascular cognitive impairment, and the current and future need for drugs and devices for restorative therapy after stroke, among other things.
- Cardiology Genomics
- Nursing
- Genomics
- Dementias Clinical Trials
- Episodic & Paroxysmal Disorders
- Cardiology Genomics
- Nursing
- Featured Meeting - Specialty page
- Exclusive Article - For home page
- Neurology Genomics
- Dementias
- Neurology Clinical Trials
- Neurology
- Episodic & Paroxysmal Disorders
During an invited symposium at the 2013 International Stroke Conference, investigators shared their thoughts on key areas in stroke research that will shape the field in a number of ways.
WORLDWIDE BURDEN: NEED FOR BETTER PREVENTION OVER A LIFETIME
Lewis B. Morgenstern, MD, University of Michigan, Ann Arbor, Michigan, USA, opened the session with an epidemiological look at stroke mortality and its global health burden. Citing a recently published article that looked at 20 years of mortality data from 1990 to 2010 [Lozano R et al. Lancet 2012], he highlighted that stroke has remained the second most common cause of death worldwide and that the mortality rate has increased by 26% over 20 years. Even more sobering, he said, is the 177% increase over 20 years in the global years of life lost due to stroke.
Although stroke incidence and mortality are declining in the West, the burden of stroke remains robust and unchanged or increasing for the poor and minorities. This indicates that it is increasingly becoming a disease of the poor and underserved populations.
Given the disparities, Dr. Morgenstern urged the use of pragmatic and cost-effective ways to implement interventions, such as educating at-risk populations of the signs of stoke and of when to call 911. He also urged people to think globally and to act locally to reduce stroke disparities by, among other things, participating in research and advocacy as well as in stroke prevention and preparedness education for underserved populations.
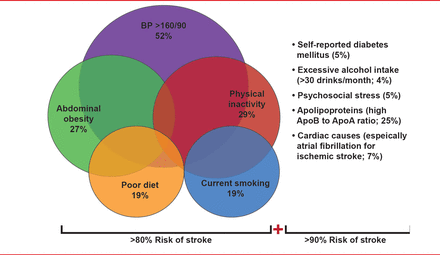
Valery Feigin, MD, PhD, AUT University, Auckland, New Zealand, expanded on Dr. Morgenstern's presentation, discussing mechanisms in which to reduce the global burden of stroke through prevention. Citing data from the INTERSTROKE study [O'Donnell MJ et al. Lancet 2010], he highlighted that >90% of strokes are caused by modifiable risk factors and are therefore preventable (Figure 1).
Risk Factors for Ischemic Stroke and Intracerebral Hemorrhagic Stroke
AF=atrial fibrillation; ApoA1=apolipoprotein A1; ApoB=apolipoprotein B; BP=blood pressure; DM=diabetes mellitus. Reproduced with permission from V Feignin, MD, PhD.
Although Prof. Feigin said that identifying and managing individuals with high risk factors is one approach for stroke prevention, he recommended a population-based approach as the primary approach as it is more cost-effective and because most strokes occur in people with only a modest increase in stroke risk factors. He also emphasized that a major decrease in the incidence of stroke can be achieved with a very small shift in the distribution of risk factors across a population (Table 1).
Effect of Control of Environmental Stroke Risk Factors
Prof. Feigin therefore urged a new paradigm in stroke prevention that focuses on determining an individual's absolute risk of stroke based on the combined effect of risk factors versus correcting individual risk factors. Defining the absolute risk as the numerical probability of an event occurring within a specified period, he said that a person's risk of stroke would then be expressed as low (<10% probability of cardiovascular disease [CVD] within the next 5 years), moderate (10% to 15% risk of CVD within the next 5 years), or high risk (>15% risk of CVD within the next 5 years).
Finally, he emphasized the need to include stroke prevention in a larger prevention framework that includes other major noncommunicable diseases such as diabetes and cancer.
Emphasizing the need for improved prevention, Joanna M. Wardlaw, MD, University of Edinburgh, Edinburgh, United Kingdom, discussed future approaches that should look at an individual's risk of CVD and stroke through a life course perspective. She emphasized that features that can be linked to subsequent late-life CVD can be seen at very early ages. For example, a person's scores from intelligence testing at age 11 can predict brain size, white matter hyperintensities, and mineral deposits at age 73, all of which are related to cognition, CVD, vascular dementia, and mortality. Emphasizing the role that genetics play in who may therefore be at higher risk of developing CVD, she prioritized focusing on how the genetic code is expressed and the many not yet fully understood factors that influence genetic expression. For example, she mentioned the role of stress and its association with socioeconomic factors that are linked to an increased burden of CVD and pointed to one area to look at over the course of a person's life to assess a vulnerability that may influence later-life disease.
Ralph L. Sacco, MS, MD, University of Miami, Miami, Florida, USA, talked more about stroke genetics and the future of personalized stroke prevention. Focusing on gene discovery, he said that important findings are being made through genome-wide association studies and highlighted what is already being learned about hypertension, one of the biggest risk factors for stroke, through the discovery of unexpected and novel pathways [Ehret GB et al. Nature 2011]. Of key importance in these efforts to map the genetics of risk factors that may help individualize a preventive approach to stroke is collaboration, such as the METASTROKE Collaboration that is helping to refine phenotypes that are risk factors for stroke (Figure 2) [Traylor M et al. Lancet Neurol 2012].
Genetic Risk Factors for Ischemic Stroke Subtypes
Reproduced from Traylor M et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE Collaboration): a meta-analysis of genome-wide association studies. Lancet Neurology Nov 2012;11(11):951–962.
Additional collaborative efforts to refine these phenotypes even further are underway through the NOMAFS family study of stroke risk and carotid atherosclerosis by Sacco and colleagues [Ethn Dis 2007] that includes 108 families and 1500 Caribbean Hispanics. Results from this study and those of other family studies known as “life after linkage” are expected to further refine phenotypes. According to Dr. Sacco, once phenotypes are refined, further work can be done on identifying metabolic pathways. These pathways can then be investigated through translational animal models and in clinical trials of new agents to tailor stroke prevention treatments.
CURRENT STATE OF RESEARCH: CLINICAL STUDIES IN STROKE
Steven J. Warach, MD, PhD, Seton/UT Southwestern Clinical Research Institute, Austin, Texas, USA, provided an overview of the state of clinical research in stroke by describing a transition from clinical impressions to a more rigorous, biologically-based clinical science. He used the example of transient ischemic attack (TIA) to illustrate this by noting the utility of TIA as a clinical concept but one that is biologically meaningless. Past clinical trials have used TIA to select patients for inclusion as well as to determine outcomes, but he cautioned that in the era of imaging this is an anachronistic way of conducting trials and that more rigorous methods of controlling for bias and minimizing variance are needed. He said that more recent studies are using imaging for patient selection, and described one such study that used biologic case definitions to select patients for inclusion to examine the safety and efficacy of tenecteplase versus alteplase for acute ischemic stroke [Parsons M et al. N Engl J Med 2012].
Dr. Warach emphasized that the next big thing in clinical trials in the next 10 years will be the use of centralized clinical trial enrollment and assessment using telemedicine, telestroke, teleradiology, and the use of smart phones. Instead of many investigators conducting clinical trials, he said that patient selection for and assessing clinical outcomes of trials will be done by a centralized group of people.
In a talk that focused on current clinical trials for carotid artery disease, Thomas G. Brott, MD, Mayo Clinic, Jacksonville, Florida, USA, also outlined the need in future trials to use biological case definitions and urged participants to use centralized databases, such as the National Institute of Neurological Disorders and Stroke, National Library of Medicine, Food and Drug Administration and Critical Path Institute, and others such as Centers for Medicare and Medicaid Services and Veterans Administration Medical Center, in future trials to ascertain and validate endpoints with the goal to improve efficiency and cost.
Dr. Brott also emphasized the need for a better understanding of which patients to include in carotid artery trials, and described current investigation into multiple biomarkers of unstable carotid plaque as well as molecular processes underlying plaque vulnerability that is ongoing but has not yet reached the bedside. Although the markers of risk are improving, he says more investigation is needed for these markers to be useful in selecting which patients should be entered in clinical trials.
Finally, Dr. Brott spoke about the need in future trials to begin looking at cognitive functions and networking of brains, and he cited 2 recently published trials that have begun to address this [Cheng HL et al. Stroke 2012; Willaert WI et al. Br J Surg 2012]. He also urged the need for improved interventional treatments.
NEED TO PAY ATTENTION TO COGNITIVE DEFICITS: RECOGNITION OF VASCULAR COGNITIVE IMPAIRMENT
Citing a study in which the American Heart Association/American Stroke Association urged recognition of and attention to vascular cognitive impairment (VCI) [Gorelick PB et al. Stroke 2011], Lenore J. Launer, PhD, National Institute on Aging, Bethesda, Maryland, USA, provided a brief primer on VCI, defining it as “the spectrum of cognitive disorders from vascular mild cognitive impairment to vascular dementia and to vascular comorbidity in Alzheimer's disease” and discussing its current assessment by neuropsychologic evidence that shows impairment in a number of cognitive domains that are linked to evidence of vascular disease. She emphasized that the evidence shows a high frequency of VCI because of the high prevalence of silent infarcts (occurring in about 25% to 30% of people) and white matter damage that is evident on magnetic resonance imaging in up to 95% of older people. Although the current definitions of VCI are lacking and not standardized, she urged participants to pursue research into understanding the physiologic mechanisms underlying the interaction between neurodegeneration and vascular pathology as well as developing new methods to better detect structural and functional changes in the brain. For example, she highlighted the need for improved detection of vascular pathology in the gray matter such as microinfarcts, microhemorrhages, and amyloid deposition, as well as the need to identify biomarkers for vascular-cognitive links.
Finally, Dr. Launer highlighted the importance of screening and the need to identify people at risk of VCI such as older persons, those with vascular disease such as diabetes, hypertension, atrial fibrillation, stroke, and kidney disease (she emphasized, however, that not everyone with vascular disease has cognitive impairment), as well as younger people in whom vascular disease is developing at ages younger than the current generation.
CURRENT AND FUTURE RESTORATIVE TREATMENTS
Randolph J. Nudo, PhD, Kansas University Medical Center, Kansas City, Kansas, USA, spoke about the current and future need for drugs and devices for restorative therapy after stroke. He said that it has now been over 2 decades since a landmark study by Chollet et al. [Ann Neurol 1991] that provided imaging evidence of the plasticity of the brain after stroke. Animal studies on brain plasticity [Nudo RJ et al. Science 1996; Dancause N et al. J Neurosci 2005; Liauw J et al. J Cereb Blood Flow Metab 2008; Overman JJ et al. Proc Natl Acad Sci USA 2012] have helped deepen an understanding of the mechanisms underlying regeneration of the brain and are now pointing to the potential for restorative therapies. A new focus of research is on defining treatment targets as well as the window in which therapies are most effective. Research by Carmichael and colleagues [Exp Neurol 2005] showed that genes are up- or down-regulated during a certain window of time after injury, indicating that restorative processes have a relatively long time course on the order of perhaps weeks to months.
Dr. Nudo talked about the potent effect of behavioral experiences on recovery, and the importance of behavior in developing neurotechnologies that increasingly rely on neuromodulation. Along with existing therapeutic technologies including deep brain stimulation and cochlear implants, he described newer technologies under development such as noninvasive modulation, including transcranial magnetic stimulation and transcranial direct-current stimulation [Sharma N, Cohen LG. Dev Psychobiol 2012], as well as the brain-machine interface in which neural signals are recorded from the brain as output commands to control external devices such as robotic systems [Hochberg LR et al. Nature 2012]. He also discussed advanced closed-loop systems that combine neural recording, signal processing and microstimulation in a single device for closed-loop operation with the intent to induce plasticity [Azin M et al. IEEE Trans Biomed Eng 2011].
Robotic workers are the solution to providing more exercise therapy with fewer people and more hours.
According to Joel Stein, MD, NewYork-Presbyterian Hospital, New York, New York, USA, robotics are the next big thing in stroke rehabilitation and recovery. Robots are a way of delivering exercise and task practice therapy, which remains the current technique that is most effective in enhancing recovery. He emphasized that future approaches to stroke rehabilitation will incorporate exercise and task practice, and that, with the shortage of people to do this work, robotic workers are the solution to providing more exercise therapy with fewer people and more hours.
An advantage of using robotics is their ability to deliver treatments repetitively and consistently without experiencing fatigue as occurs with humans delivering these treatments. Another advantage is making therapy more interesting to patients, such as with the use of gaming.
Citing a multicenter study conducted by the Department of Veterans Affairs of 127 chronic hemiparetic stroke survivors assigned to 12 weeks of intensive robot-assisted therapy (n=49), intensive therapist-assisted therapy (n=50), or usual care (n=28), he said the data show that the effects of intensive robot-assisted therapy were modest but similar in outcome to those achieved with intensive therapist-assisted therapy [Lo AC et al. N Engl J Med 2010].
Dr. Stein briefly described a couple of advances in robotic therapy including the exoskeletal work-station device that provides greater movement in the arm, as well as wearable devices such as a powered knee brace that allows patients to interact in a more community-based setting.
ADVANCES COMING IN THE NEAR FUTURE: NEUROPROTECTION
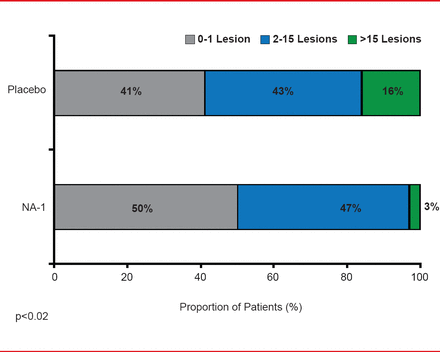
Along with increasing advances in neurotechnology and robotics, another advance that may be just on the horizon is the feasibility of reducing tissue damage after early onset of stroke through neuroprotection. Michael Tymianski, MD, PhD, Toronto Western Hospital Research Institute, Toronto, Ontario, Canada, walked participants through the evidence that shows early neuroprotection within 3 hours is possible and with further investigation may also be practicable. He presented data from studies that suggest that neuroprotection likely has a window of efficacy of about 3 hours after stroke [Bråtane BT et al. Stroke 2012; Cook DJ et al. Nature 2012] that is similar to the window of efficacy demonstrated for reperfusion [Hacke W et al. N Engl J Med 2008; NINDS rt-PA Study Group. N Engl J Med 1995]. Data in nonhuman primates showed that the use of a PSD-95 inhibitor administered within 3 days after stroke onset provided biological neuroprotection [Cook DJ et al. Nature 2012]. The first evidence to show that biological neuroprotection is possible in aged humans of both genders with demographics similar to stroke victims comes from a Phase 2 clinical trial of patients with iatrogenic stroke who were administered a PSD-95 inhibitor after endovascular aneurysm repair. This 2012 trial showed that there were fewer lesions per person in the PSD-95 group compared with the placebo group (p=0.012) [Hill MD et al. Lancet Neurol 2012].
With the possibility of neuroprotection established based on the availability of an effective drug such as PSD-95 inhibitor, Prof. Tymianski said the second question to address is whether neuroprotection is practicable. Based on the current evidence, he said that there is no good evidence that neuroprotection is possible when administered more than 4 hours after stroke onset (Table 2).
Prof. Tymianski also emphasized that neuroprotectants, like reperfusion therapies, should only be used as emergency drugs administered in a hospital setting and only if the drugs are safe. Whether neuroprotection is practicable, he said, will rely on evidence from a clinical trial. He proposed a trial called Field Randomization of NA-1 Therapy in Early Responders [FRONTIER] that will be a prehospital trial in which a drug is started before arrival to the emergency room and treatment will not interfere with institutional practices that include reperfusion therapies. Such a trial, he said, could be completed in 3 years, bringing the practicability of neuroprotection within clinical reach soon.
Neuroprotection Trials Over the Past Decade.
- © 2013 MD Conference Express®