Summary
Bisphosphonates provide substantial protection against fractures and strong evidence shows this protection occurs early, within weeks or months of initiation of therapy, and the benefit persists, although does not increase, over time. Other management strategies for osteoporosis discussed in this article are combinations of drugs that further contribute to bone fragility, as well as prevention strategies such as calcium, vitamin D, and bisphosphonates.
- Metabolic Bone Disease
- Metabolic Bone Disease
- Rheumatology
- Exclusive Article - For home page
Bisphosphonates provide substantial protection against fractures and strong evidence shows this protection occurs early, within weeks or months of initiation of therapy, and the benefit persists, although does not increase, over time. The overall risk:benefit ratio of bisphosphonates is favorable at 10 years and especially to 5 years, according to Michael McClung, MD, Oregon Osteoporosis Center, Portland, Oregon, USA.
Vertebral fractures were reduced by 60% to 70%, and even in patients on long-term glucocorticoid therapy fractures were reduced by 70% by 1 year with bisphosphonates [McClung M et al. Am J Med 2013]. Multiple vertebral fractures were reduced by 75% to 96%, hip fractures by 40% to 50%, and nonvertebral fractures by 20% to 35%.
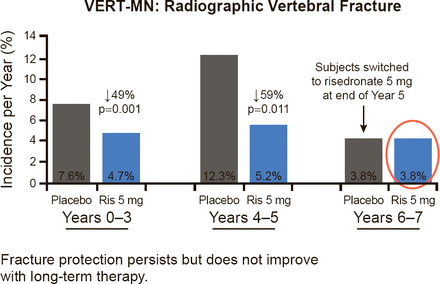
The early benefit with the bisphosphonate risedronate in two different studies showed response as early as 6 to 12 months in nonvertebral and hip fractures, respectively [Harrington JT et al. Calcif Tissue Int 2004; McClung MR et al. N Engl J Med 2001]. An example of the persistent benefit with treatment, from the VERT-MN study with risedronate, is shown in Figure 1. However, Dr. McClung noted that, while there does not appear to be improvement in Years 6 to 7, that does not mean that the bisphosphonate is not working. The benefits are persistent, but not necessarily progressive.
Fracture Protection With Bisphosphonates Is Persistent
Reproduced from Harrington JT et al. Risedronate Rapidly Reduces the Risk for Nonvertebral Fractures in Women with Postmenopausal Osteoporosis. Calcif Tissue Int 2004;74(2)129–135. With permission from Springer-Verlag.
Bisphosphonates are generally well tolerated and were very safe in randomized clinical trials. Some side effects that could be considered “nuisance problems,” said Dr. McClung, include upper gastrointestinal intolerance that was rarely seen in some 50 clinical trials but often limits acceptance and persistence of bisphosphonate treatment. Others are acute phase reaction after intravenous (IV) or high-dose oral therapy that is flu-like and short-lived; bone and muscle pain that was not seen in clinical trials and has an unknown incidence and cause; inflammatory eye problems, including uveitis and iritis; renal impairment and failure with IV therapy, that was not seen in clinical trials with careful attention to pretreatment renal function and volume status; and anaphylactic reaction that is rare with an unknown incidence [McClung M et al. Am J Med 2013].
Major safety concerns with bisphosphonates are atypical femoral fracture (AFF), atrial fibrillation, and esophageal cancer. The United States Food and Drug Administration (FDA) concluded there was no evidence for a link between bisphosphonates and atrial fibrillation, which was seen in only one of the two HORIZON studies with zoledronic acid [Black DM et al. N Engl J Med 2007]. Although a series of cases of patients with esophageal cancer were reported [Wysowski DK. N Engl J Med 2009], most observational studies have reported no risk or decreased risk of esophageal cancer [McClung M et al. Am J Med 2013; Cardwell CR et al. JAMA 2010]. One study reported an increased risk of esophageal cancer in patients with 10 or more prescriptions of oral bisphosphonates over 3 years, and the absolute risk was calculated to be 1 per 1000 population over 5 years [Green J et al. BMJ 2010]. The FDA concluded there was no association between oral bisphosphonates and an increased risk of esophageal cancer. However, bisphosphonates should be avoided in patients with Barrett's esophagus.
Regarding AFF, in a Swedish cohort of nearly 12,800 women there was a suggestion of an increase in the absolute risk, (5 cases per 10,000 patient-years; OR, 1.3; 95% CI, 1.1 to 1.6) [Schilcher J et al. N Engl J Med 2011]. The risk of AFF is related to duration of bisphosphonate treatment. The risk of AFF decreased rapidly after drug withdrawal in this cohort, ∼70% in each of the first 2 years (OR, 0.28; 95% CI, 0.21 to 0.38). In a cohort of 1,835,116 patients in the United States, the risk of duration-dependent AFF was 1.78 per 100,000 patient-years in the first 2 years of treatment and 113.1 per 100,000 patient-years during Years 8 to 9.9 of treatment [Dell RM et al. J Bone Miner Res 2012].
In patients with moderate fracture risk, a drug holiday of 1 to 3 years can be considered after 3 to 5 years of bisphosphonate treatment, because of its persistent protection, and the risk of AFF is reduced [Whitaker M et al. N Engl J Med 2012]. However, in patients at high risk of spine fracture, continuing treatment through 10 years is justified.
Regarding calcium and vitamin D, a reasonable recommendation for their daily intake from food and supplements based on current evidence is 800 to 1200 mg and 800 to 2000 IU, respectively, stated Dr. McClung.
MEDICATION-INDUCED OSTEOPOROSIS AND FRACTURES
Many common drugs prescribed by rheumatologists contribute to increased fracture risk (Table 1). Mary Beth Humphrey, MD, PhD, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA, stated that more studies are needed to determine how combinations of these drugs further contribute to bone fragility, and that prevention strategies include calcium, vitamin D, and bisphosphonates are needed.
Drugs Known to Increase Bone Fracture Risk
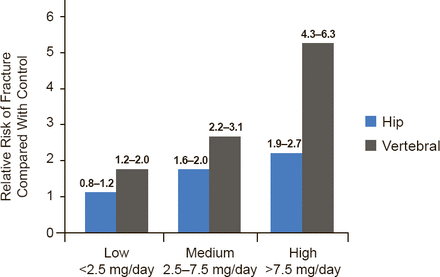
Glucocorticoid (GC) use is highest among older adults (3.5% of men aged ≥80 years; 2.7% of women aged 70 to 79 years), yet concomitant use of bisphosphonates in all patients taking a GC is low at only 8.6% [Overman RA et al. Arthritis Care Res (Hoboken) 2013]. The relative risk of hip and vertebral fracture with GC use is shown in Figure 2.
Risk of Hip and Vertebral Fracture With Glucocorticoid Use
n=244,000 cases and controls
Reproduced with permission from MB Humphrey, MD, PhD.
The American College of Rheumatology recommendations [Grossman JM et al. Arthritis Care Res (Hoboken) 2010] for postmenopausal women and men aged ≥50 taking a GC ≥3 months is bisphosphonate treatment for patients at low risk (World Health Organization Fracture Risk Assessment Score [FRAX] <10%) on prednisone ≥7.5 mg or moderate risk (FRAX 10% to 19%) on any GC dose, and a bisphosphonate or teriparatide for high-risk (FRAX >20%) patients on any GC dose. For premenopausal women with childbearing potential and men aged <50 years taking a GC for 1 to 3 months, there are insufficient data for a recommendation, and a bisphosphonate or teriparatide is recommended for patients taking prednisone >7.5 mg for >3 months. For premenopausal women without childbearing potential and men aged <50 years taking prednisone >5 mg for 1 to 3 months a bisphosphonate is recommended, and a bisphosphonate or teriparatide is recommended for any GC dose taken for >3 months.
Proton pump inhibitors have a modest relative risk of ∼1.5 for fractures that is dose and duration dependent, which is further increased with concomitant GC use [Kwok CS et al Bone 2011; Pouwels S et al. Osteoporos Int 2011]. Selective serotonin reuptake inhibitors were associated with a relative risk of 1.72 for fracture in a meta-analysis of 12 studies [Wu Q et al. Osteoporos Int 2012].
Anticonvulsant drugs are thought to increase vitamin D metabolism and alter calcium absorption, and directly inhibit osteoblasts in vitro. In one study of patients aged ≥50 years taking an antiepileptic drug, the adjusted OR ranged from 1.24 with clonazepam (95% CI, 1.05 to 1.47) to 1.91 with phenytoin (95% CI, 1.58 to 2.30) [Jetté N et al. Arch Neurol 2011]. Valproic acid was not associated with an increased fracture risk (OR, 1.10; 95% CI, 0.70 to 1.72) in this study.
Heparin is associated with dose- and duration-dependent bone loss. The risk of osteoporosis was increased with long-term use of unfractionated heparin, with a fracture rate of 2% to 3%; vertebral fractures being most common. The risk of osteoporosis, although present, was substantially lower with low molecular weight heparin [Rajgopal R et al. Thromb Res 2008]. Warfarin was shown to be associated with increased fracture risk in a population-based retrospective cohort, with 12 months of treatment being an independent predictor of vertebral (p=0.009) and rib (p=0.02) fracture [Caraballo PJ et al. Arch Intern Med 1999].
Thiazolidinediones, used to treat type 2 diabetes and commonly seen in the rheumatology clinic, were shown to increase fracture risk in women but not men with long-term use in one meta-analysis [Loke YK et al. CMAJ 2009]. At 4 years of treatment with a thiazolidinedione, treatment of ∼50 to 100 patients induced one fracture [Dormuth CR et al. Arch Intern Med 2009]. Patients on multiple drugs may require coordination between the endocrinologist and rheumatologist.
Calcineurin inhibitors, including tacrolimus and cyclosporine, are associated with dose- and time-dependent bone loss, and ∼46% of users will have a hip fracture compared with 20% of nonusers [Mazzantini M et al. Clin Exp Rheumatol 2007].
Dr. Humphrey stated that additional studies are needed to determine how the combinations of these medications further contribute to bone fragility in patients.
- © 2013 MD Conference Express®