Summary
A prespecified integrated analysis of two Phase 3 clinical trials demonstrates that the monoclonal antibody ustekinumab reduces radiographic progression of joint disease in patients with active psoriatic arthritis. This article presents the integrated analysis of the two Phase 3 Multicenter, Randomised, Double-Blind, Placebo-Controlled Trials of Ustekinumab, a Fully Human Anti-IL-12/23p40 Monoclonal Antibody, Administered Subcutaneously, in Subjects With Active Psoriasis Arthritis [PSUMMIT I and II; NCT01009086; NCT01077362].
- Arthritis
- Rheumatology Clinical Trials
- Rheumatology
- Arthritis
- Rheumatology Clinical Trials
A prespecified integrated analysis of two Phase 3 clinical trials demonstrates that the monoclonal antibody ustekinumab reduces radiographic progression of joint disease in patients with active psoriatic arthritis. Iain B. McInnes, PhD, University of Glasgow, Glasgow, Scotland, presented the integrated analysis of the two Phase 3 Multicenter, Randomised, Double-Blind, Placebo-Controlled Trials of Ustekinumab, a Fully Human Anti-IL-12/23p40 Monoclonal Antibody, Administered Subcutaneously, in Subjects With Active Psoriasis Arthritis [PSUMMIT I and II; NCT01009086; NCT01077362].
Ustekinumab is a human monoclonal antibody against the p40 subunit of interleukins (IL)-12 and −23. The IL-23/IL-17 axis mediates pathways that have the potential to drive inflammation and matrix destruction, said Prof. McInnes.
The 615 patients enrolled in PSUMMIT I had inadequate response to methotrexate and no exposure to tumor necrosis factor (TNF)-α inhibitors. Prior anti-TNF-α therapy was permitted in PSUMMIT II, in which 312 patients were enrolled. In PSUMMIT II, 70% of patients had discontinued TNF-α inhibitors for lack of efficacy or intolerance; 25% had received ≥3 prior anti-TNF-α therapies. Therapies at baseline are depicted in Table 1. The integrated analysis therefore included 927 patients with disease activity despite prior treatment with alternative agents.
In the trials, patients were randomized to receive ustekinumab 45 mg, 90 mg, or placebo at Weeks 0 and 4 and then every 12 weeks [McInnes JB et al. Lancet 2013; Ritchlin CT et al. Arthritis Rheum 2012 (abstr 2557)]. In each study, patients with no response to placebo (defined as <5% improvement in tender and swollen joint count from baseline) at Week 16 were crossed over to ustekinumab 45 mg. All remaining patients randomized to placebo crossed over at Week 24 to ustekinumab 45 mg. Patients randomized to ustekinumab 45 mg who had no response (as defined above) had their dose of ustekinumab increased to 90 mg, starting at Week 16. Radiographic progression was assessed in the hands and the feet by the change from baseline to Week 24 in total psoriatic arthritis modified van der Heijde-Sharp (vdHS) scores.
Prior Use of Therapies at Baseline in PSUMMIT I and PSUMMIT II
Linear extrapolation to Week 24 was performed if there was a baseline x-ray available and a second x-ray performed before Week 24; if data were insufficient for linear extrapolation (ie, only 0 or 1 available radiographs), the median of the change in vdHS derived from all subjects within the same methotrexate stratification group at the missing visit was assigned.
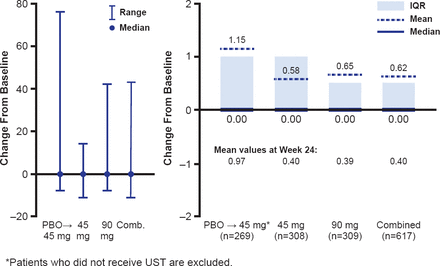
In the integrated analysis, at Week 24, patients randomized to ustekinumab 45 and 90 mg had a mean change from baseline in total vdHS score of 0.40 and 0.39, respectively, compared with a mean change of 0.97 for patients receiving placebo (p=0.017 and p<0.001, respectively). The favorable effect of ustekinumab on radiographic progression continued to Week 52 (Figure 1).
When evaluated individually, results from PSUMMIT I were consistent with the prespecified integrated analysis (significant inhibition of structural damage at Week 24 for both ustekinumab doses). The effect of ustekinumab on inhibiting progression of structural damage could not be discerned in the smaller PSUMMIT II study, which had a high proportion of dropouts in the placebo group. Different rates of imputation for missing data could have disproportionately altered progression rates in PSUMMIT II, potentially obscuring any true difference in radiographic progression between groups, explained Prof. McInnes.
Change From Baseline in Modified Total vdHS Score at Week 52
ITT=intention-to-treat; PBO=placebo; UST=ustekinumab; vdHS=van der Heijde-Sharp.
Reproduced with permission from IB McInnes, PhD.
- © 2013 MD Conference Express®