Summary
This article discusses discussed major advances in the treatment of human epidermal growth factor 2 (HER2)-positive breast cancer in the past decade, the current state of adjuvant therapy for HER2-positive breast cancer, as well as the therapeutic implications of neoadjuvant therapy for HER2-positive breast cancer.
- Breast Cancer
- Adjuvant/Neoadjuvant Therapy
- Oncology Genomics
Nancy U. Lin, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, USA, discussed major advances in the treatment of human epidermal growth factor 2 (HER2)-positive breast cancer in the past decade. Including the expansion of HER2-directed therapy into earlier disease stages, continuation of HER2-directed therapy after progression, and the use of combined targeted approaches.
Key trials of first-line therapy for HER2-positive metastatic breast cancer (MBC) have demonstrated that trastuzumab plus chemotherapy is superior to chemotherapy alone [Slamon DJ et al. N Engl J Med 2001], HER2-directed therapy plus endocrine therapy improves outcomes compared with endocrine therapy alone [Kaufman B et al. J Clin Oncol 2009; Johnston S et al. J Clin Oncol 2009], single-agent chemotherapy plus trastuzumab is comparable to a chemotherapy doublet plus trastuzumab [Forbes JF et al. J Clin Oncol 2006 (suppl; abstr LBA516); Pegram M et al. J Clin Oncol 2007 (suppl; abstr LBA1008)], and several chemotherapy agents are effective when paired with trastuzumab [Burstein HJ et al. Cancer 2007; Andersson M et al. J Clin Oncol 2010].
Recent research has focused on new HER2-targeted agents. Pertuzumab, which binds to different regions on HER2 than trastuzumab, signficantly prolonged progression-free survival (PFS) in the CLEOPATRA trial of first-line pertuzumab plus trastuzumab plus docetaxel (18.5 months) versus trastuzumab plus docetaxel (12.4 months; HR for progression or death, 0.62; 95% CI, 0.51 to 0.75; p<0.001) [Baselga J et al. N Engl J Med 2012].
Study results have confirmed that HER2 is a valid target after progression on trastuzumab, with improved PFS [Geyer CE et al. N Engl J Med 2006; von Minckwitz G et al. J Clin Oncol 2009; Blackwell KL et al. J Clin Oncol 2010] and overall survival (OS) [Blackwell KL et al. J Clin Oncol 2012].
Novel HER2-directed agents under investigation include trastuzumab emtansine (T-DM1), neratinib, afatinib, MM-302, ertumaxomab, and AE37. T-DM1 is trastuzumab linked to DM1, a potent cytotoxic agent that selectively delivers DM1 to HER2 positive tumor cells. A first-line study found that median PFS was prolonged with T-DM1 versus trastuzumab plus docetaxel (14.2 vs 9.2 months; HR, 0.59; 95% CI, 0.36 to 0.97; p=0.035) [Hurvitz S et al. Eur J Cancer 2011 (suppl 1; abstr LBA3)]. The EMILIA study reported superior outcomes with T-DM1 versus lapatinib plus capecitabine for PFS (9.6 vs 6.4 months; HR for progression or death from any cause, 0.65; 95% CI, 0.55 to 0.77; p<0.001) and median OS (30.9 vs 25.1 months; HR for death from any cause, 0.68; 95% CI, 0.55 to 0.85; p<0.001) [Verma S et al. N Engl J Med 2012].
Tremendous progress has been made with the approval of trastuzumab, lapatinib, and pertuzumab. Pertuzumab plus trastuzumab plus a taxane has been approved for first-line therapy, and T-DM1 approval is expected in 2013. HER2-directed therapy provides benefit for third-line treatment and beyond. Trials have shown that HER2 remains a valid target after progression on trastuzumab, and combinations of HER2-directed therapies can overcome trastuzumab resistance. However, HER2-positive MBC is still largely incurable and relapses can occur despite adjuvant therapy.
Adjuvant Therapy
Trastuzumab added to adjuvant chemotherapy has been the standard therapy for early HER2-positive breast cancer since 2005. Karen Gelmon, MD, University of British Columbia, Vancouver, British Columbia, Canada, discussed the current state of adjuvant therapy for HER2-positive breast cancer.
The PHARE and HERA trials that investigated trastuzumab duration found no significant difference in disease-free survival with 6 versus 12 months at 4 years of follow-up (84.9% vs 87.8%; HR, 1.28; 95% CI, 1.05 to 1.56; p=0.29; noninferiority not established) or 2 years versus 1 year with 8 years of follow-up (75.8% vs 76.0%; unadjusted HR, 0.99; 95% CI, 0.85 to 1.14; p=0.8588) of treatment, respectively [Pivot X et al. Ann Oncol 2012 (suppl 9; abstr LBA5); Goldhirsch A et al. Ann Oncol 2012 (suppl 9; abstr LBA6)]. The SOLD [NCT00593697] and SHORT-HER [NCT00629278] trials are investigating 9 weeks versus 12 months of combination therapy with trastuzumab.
The ALTTO [NCT00490139] trial is evaluating chemotherapy plus a year of trastuzumab or lapatinib, both agents sequentially, or both agents concurrently. The lapatinib monotherapy arm was discontinued when it was shown to be inferior, and final trial results are pending. Gelmon et al. [J Clin Oncol 2012 (suppl; abstr LBA671)] found that median PFS was shorter with lapatinib (9.0 months) versus trastuzumab (13.7 months) when both were combined with taxane-based therapy, and the centrally confirmed HER2-positive subgroup had an HR of 1.48 (95% CI, 1.15 to 1.92; p=0.003). The NeoALTTO trial [NCT00553358] demonstrated improved pathologic complete response (pCR) with lapatinib plus trastuzumab (51.3%) compared with trastuzumab alone (29.5%; p=0.0001) [Baselga J et al. Lancet 2012].
Results of the AVEREL study [Gianni L et al. SABCS 2011 (abstr S4–8)] as assessed by independent review committee demonstrated a statistically significant benefit for the addition of bevacizumab to trastuzumab; the BETH Study [NCT00625898] has not yet reported results for the adding bevacizumab to chemotherapy plus trastuzumab. The CLEOPATRA trial found significantly increased PFS with the addition of pertuzumab to trastuzumab plus docetaxel [Baselga J et al. N Engl J Med 2012]. The ongoing APHINITY [NCT01358877] trial is evaluating chemotherapy plus one year of trastuzumab with or without pertuzumab.
Cardiac toxicity remains the most common long-term concern with trastuzumab treatment. Another issue is whether all HER2-positive cancers are the same or whether treatment can be tailored according to molecular features. Individualized treatment has the potential to decrease toxicity, improve quality of life, and improve outcomes.
Neoadjuvant Therapy
Neoadjuvant therapy provides a unique platform to assess tumor response to treatment, obtain serial tumor biopsies, and understand tumor biology. Mothaffar Rimawi, MD, Baylor College of Medicine, Houston, Texas, USA, discussed the therapeutic implications of neoadjuvant therapy for HER2-positive breast cancer.
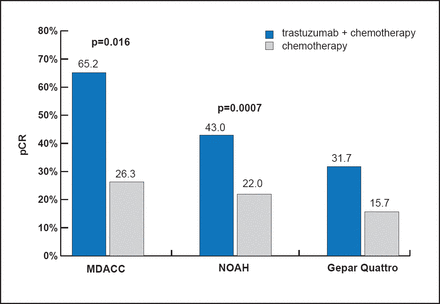
Three neoadjuvant therapy trials demonstrated improved pCR with the addition of trastuzumab to chemotherapy versus chemotherapy alone (Figure 1). Although the pCR rates were almost double with trastuzumab, considerable resistance to trastuzumab was evident. Lapatinib is a dual inhibitor of the HER2 and EGFR tyrosine kinases that should potently signal downstream of both their homo- and heterodimeric forms. Interestingly, the GeparQuinto trial reported a pCR rate of 30.3% with chemotherapy plus trastuzumab compared with 22.7% with chemotherapy plus lapatinib (OR, 0.68; 95% CI, 0.47 to 0.97; p=0.04) [Untch M et al. Lancet Oncol 2012]. Based on these data, Dr. Rimawi concluded that targeting HER2 with only one inhibitor is not the optimal strategy.
BREAST-Q conceptual framework.
Buzdar Au et al. J Clin Onc 2005; Gianni L et al. Lancet 2010; Untch et al. J Clin Onc 2010.
NSABP B-41 reported 52.5% (p=0.9) pCR for its trastuzumab arm, 53.2% for its lapatinib arm, and 62.0% for its lapatinib plus trastuzumab arm (p=0.075) [Robidoux A et al. J Clin Oncol 2012 (suppl; abstr LBA506)]. The NeoSphere trial reported pCR rates of 45.8% with trastuzumab and pertuzumab plus docetaxel compared with 29.0% with trastuzumab plus docetaxel (p=0.0141); pCR was 24.0% with pertuzumab plus docetaxel and 16.8% with trastuzumab and pertuzumab [Gianni L et al. Lancet Oncol 2012]. The results of these studies indicate that multitargeted therapy inhibits the HER pathway more potently when combined with chemotherapy.
Dr. Rimawi said that multitargeted approaches to blockade of HER2 signaling have improved outcomes in HER2-positive breast cancer. Some patients may not need chemotherapy, but further biomarker research and studies are needed to determine if they can be identified. An important question is whether targeting ER is important.
- © 2013 MD Conference Express®