Summary
This article reviews data on the feasibility and accuracy of sentinel node biopsy after neoadjuvant chemotherapy, and discusses the latest advances in breast reconstruction following mastectomy, as well as the use of preoperative MRI for the detection of multicentric disease in women diagnosed with breast cancer.
- Adjuvant/Neoadjuvant Therapy
- Breast Cancer
- Radiology
Sentinel Node Biopsy: Before or After Neoadjuvant Chemotherapy?
Neoadjuvant chemotherapy (NACT) has been found to have equivalent outcomes to adjuvant chemotherapy for breast cancer in terms of disease-free survival (DFS) and overall survival (OS). Additionally, NACT has been shown to increase the rate of breast-conserving surgery (BCS) without adversely effecting DFS or OS. However, the role of sentinel node biopsy (SNB) after NACT that may potentially sterilize the axilla remains unclear. Eleftherios Mamounas, MD, MPH, Northeastern Ohio Medical University, Rootstown, Ohio, USA, reviewed data on the feasibility and accuracy of SNB after NACT.
Pooled data from single-institution studies of SNB after NACT show considerable variability in sentinel lymph node (SLN) identification and false-negative rates due to small numbers of subjects. A meta-analysis of 24 single and multicenter studies of SNB after NACT in 1799 subjects with early-stage breast cancer reported a pooled SLN identification rate of 89.6% (95% CI, 86.0 to 92.3) with moderate heterogeneity and a false-negative rate of 8.4% (95% CI, 6.4 to 10.9) with no significant heterogeneity [Kelly AM et al. Acad Radiol 2009]. The authors concluded that SNB is a reliable tool for planning treatment after NACT.
A study of SNB after NACT (n=575) versus SNB upfront (n=3171) reported SLN identification rates of 97.4% versus 98.7% (p=0.017), false-negative rates of 5.9% versus 4.1% (p=0.39), and positive node rates of 12.7% versus 19.0% (T1; p=0.2), 20.5% versus 36.5% (T2; p<0.0001), and 30.4% versus 51.4% (T3; p=0.04) [Hunt KK et al. Ann Surg 2009]. The investigators concluded that SNB after NACT is as accurate as SNB before NACT, results in fewer positive SLNs, and decreases unnecessary axillary dissections.
Proponents of SNB before NACT contend that this approach provides information on SLN status without the confounding effects of NACT and that SLN patients can avoid axillary dissection. Dr. Mamounas argued that although this strategy may benefit patients with negative SLNs, it is not useful for most NACT candidates. This approach also does not take advantage of the downstaging benefit of NACT on nodes and requires two surgical procedures for most patients.
Dr. Mamounas concluded that SNB before NACT does not offer clinical advantages and reduces the number of patients who could benefit from the down-staging effect of NACT on the axillary nodes. SNB after NACT is feasible and accurate with similar performance as SNB before NACT. By performing SNB after NACT, up to 40% of patients who present with involved axillary nodes may be spared axillary dissection. Caution is required for patients who present with clinically or pathologically involved nodes before NACT.
Implant Breast Reconstruction
Women undergoing mastectomy are increasingly choosing to undergo implant breast reconstruction. Over the past 5 years, next-generation silicone implants, acellular dermal matrices, and fat grafting have expanded the options available to patients [Pusic AL. SABCS 2012 (abstr ES6–2)]. Additionally, indications for postmastectomy radiation are increasing, with implications for long-term patient satisfaction with implant reconstruction. Andrea L. Pusic, MD, MHS, Memorial Sloan-Kettering Cancer Center, New York, New York, USA, discussed the latest advances in breast reconstruction following mastectomy.
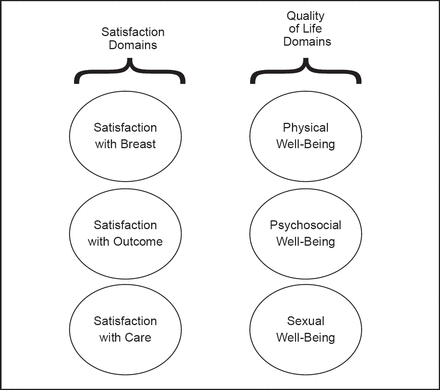
New options in breast reconstruction include the fifth generation cohesive gel implant made of cross-linked silicone and direct-to-implant surgery performed at the same time as mastectomy. Dr. Pusic emphasized the importance of patient-reported outcomes to evaluate patient satisfaction, quality of life (QoL), and adverse effects of these and other breast reconstruction methods. In collaboration with her colleagues, Dr. Pusic developed the BREAST-Q patient-reported outcome measure for use after breast surgery [Pusic AL et al. Plast Reconstr Surg 2009]. The BREAST-Q framework consists of six domains, each with a separate scale: satisfaction with breasts; outcomes; care; and physical, psychosocial, and sexual well-being (Figure 1). By measuring patient-reported outcomes, the BREAST-Q can support an evidence-based approach to breast reconstruction surgery.
BREAST-Q conceptual framework.
Reproduced with permission from Wolters Kluwer Health. Pusic A et al. Plastic and Reconstructive Surgery 2009; 124(2): 345–353.
A recent analysis of breast reconstruction trends revealed that over the last decade implant reconstruction rates have increased by an average of 11% per year, while autologous reconstruction rates have remained the same (p<0.01) [Albornoz CR et al. Plast Reconstr Surg 2013]. Another trend is the expansion of indications for radiation therapy, which negatively impacts patient satisfaction with physical, QoL, and breast reconstruction outcomes [Albornoz CR et al. ISOQOL 2012]. New devices and surgical techniques may offer benefit but should be evaluated from both the clinician and patient perspectives. The BREAST-Q can facilitate quantification of patient satisfaction, QoL, and adverse effects.
Preoperative Breast MRI
Ismail Jatoi, MD, PhD, University of Texas Health Science Center, San Antonio, Texas, USA, discussed the use of preoperative MRI for the detection of multicentric disease in women diagnosed with breast cancer. The presence of multicentric or contralateral disease is an important consideration in the selection of BCS versus mastectomy.
Multicentric breast cancer generally is detected by clinical breast examination, mammography, and ultrasound. A systematic review and meta-analysis of 50 studies showed that preoperative breast MRI detected additional disease foci in 20% of women and contralateral disease in 5.5% [Plana MN et al. Eur Radiol 2012]. Early studies have documented the presence of occult tumor foci in addition to the primary tumor in 54% to 63% of cases [Qualheim RE et al. Cancer 1957; Holland R et al. Cancer 1985; Vaidya JS et al. Br J Cancer 1996].
The possibility of multicentric disease has been a deterrent to performing BCS as an alternative to mastectomy. Two randomized trials assessed the impact of preoperative breast MRI on reoperation rates and demonstrate differing results. The COMICE trial in patients randomized to MRI versus no MRI reported reoperation rates of approximately 19% in both arms (OR, 0.96; p=0.77) [Turnbull L et al. Lancet 2010]. The MONET trial of patients with nonpalpable breast cancers randomized to MRI versus no MRI found reoperation rates of 34% versus 12% (p=0.008) [Peters NH et al. Eur J Cancer 2011].
Studies of the effect of preoperative MRI versus no MRI on local recurrences have had mixed results [Fischer U et al. Eur Radiol 2004; Hwang N et al. Ann Surg Oncol 2009; Solin LJ et al. J Clin Oncol 2008]. The Solin study found no difference between local failure rates according to the use of breast MRI at the time of initial diagnosis and evaluation for BCS with radiation.
Breast MRI is potentially useful in the following situations: screening in certain women at increased risk for breast cancer, detection of primary tumor in women with malignant axillary adenopathy and a negative mammogram, monitoring response to neoadjuvant therapy, evaluating nipple discharge, and evaluating breast implants.
Dr. Jatoi said that routine preoperative breast MRI should not be recommended because it does not reduce risk of reoperation following BCS, does not lower risk of local recurrence, may delay surgery, may increase patient anxiety, may increase risk of unnecessary breast biopsies/imaging, may unnecessarily increase mastectomy rates, and may unnecessarily increase contralateral mastectomy rates. Preoperative MRI can be useful for identifying additional lesions apart from the primary tumor. These lesions can be adequately treated with radiotherapy and systemic therapies or may have no clinical significance.
- © 2013 MD Conference Express®