Summary
Patients who develop isolated local or regional recurrences (ILRR) of breast cancer have a high risk of distant metastasis and death. The Adjuvant Chemotherapy in Treating Women Who Have Undergone Resection for Relapsed Breast Cancer (Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer) [CALOR; NCT00074152] trial investigated the impact of chemotherapy on disease-free survival and overall survival in patients with ILRR.
- Breast Cancer
- Oncology Clinical Trials
- Adjuvant/Neoadjuvant Therapy
Patients who develop isolated local or regional recurrences (ILRR) of breast cancer have a high risk of distant metastasis and death. The only prospective randomized trial of adjuvant chemotherapy in patients with ILRR reported at >11 years follow-up that patients treated with tamoxifen versus observation had significantly improved disease-free survival (DFS) but no overall survival (OS) advantage [Waeber M et al. Ann Oncol 2003]. The Adjuvant Chemotherapy in Treating Women Who Have Undergone Resection for Relapsed Breast Cancer (Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer) [CALOR; NCT00074152] trial, presented by Stefan Aebi, MD, Luzerner Kantonsspital, Lucerne, Switzerland, investigated the impact of chemotherapy on DFS and OS in patients with ILRR. The study had strong participation of the United States National Surgical Adjuvant Breast and Bowel Project, with Irene Wapnir, MD, from Stanford University, Stanford, California, USA, chairing the North American participation.
Patients with a first ILRR excised with negative or microscopically involved tumor margins and no evidence of tumor in supraclavicular lymph nodes or distant metastasis were eligible for the trial. After surgery, patients were stratified according to prior chemotherapy, estrogen receptor (ER) and/or progesterone receptor (PR) status, and location of ILRR; they were then randomized to treatment with chemotherapy (n=85) or no chemotherapy (n=77). Patients with hormone receptor-positive cancers also received endocrine therapy, and those with human epidermal growth factor receptor 2 (HER2)-positive cancers could receive HER2-directed therapy. The specific chemotherapy was chosen by investigators, but a 2-drug regimen for 3 to 6 months was recommended. Radiation therapy at ≥40 Gy was required for patients with microscopically involved margins and recommended for all patients. The primary endpoint was DFS and the secondary endpoint was OS.
Baseline characteristics were well balanced between the 2 groups. Therapies for ILRR received by patients in the chemotherapy versus no chemotherapy arms were radiation therapy (44% vs 39%), luteinizing hormone-releasing hormone (6% vs 13%), fulvestrant (0% vs 1%), tamoxifen (18% vs 18%), aromatase inhibitors (55% vs 53%), endocrine treatment for ER-positive ILRR (91% vs 92%), and HER2-directed therapies (7% vs 5%). Patients in the chemotherapy arm were treated with monotherapy (docetaxel or paclitaxel [20%], or capecitabine [11%]) or polychemotherapy (anthracycline-based [48%], anthracycline plus taxane-based [1%], or taxane-based [16%]). Therapies for ILRR in the chemotherapy arm are shown in Table 1.
Therapies for ILRR in the Chemotherapy Arm.
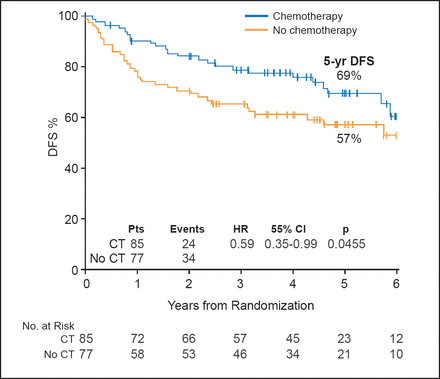
At a median follow-up of 4.9 years, patients in the chemotherapy group versus the no-chemotherapy group had significantly improved DFS (69% vs 57%; HR, 0.59; 95% CI, 0.35 to 0.99; p=0.0455; Figure 1) and OS (88% vs 76%; HR, 0.41; 95% CI, 0.19 to 0.89; p=0.02).
DFS with Chemotherapy Versus No Chemotherapy.
CT=chemotherapy; DFS=disease-free survival; Pts=patients.
Reproduced with permission from S Aebi, MD.
On multivariate analysis controlling for ILRR location, disease-free interval, ER status, and prior adjuvant chemotherapy, the results remained significant for both DFS (HR, 0.50; p=0.01) and OS (HR, 0.37; p=0.02).
Analysis by ER status showed a significant difference in DFS with chemotherapy versus no chemotherapy in ER-negative patients (DFS, 67% vs 35%; HR, 0.32; 95% CI, 0.14 to 0.73; p=0.007) but not in ER-positive patients (70% vs 69%; HR, 0.94; 95% CI, 0.47 to 1.89; p=0.87).
In the CALOR trial, adjuvant chemotherapy reduced the risk of DFS events by 41% and death by 59%. The authors concluded that adjuvant chemotherapy should be recommended for patients with completely resected isolated local or regional recurrences. The results are strongest for patients with ER-negative recurrences. Longer follow-up is needed for patients with ER-positive recurrences.
- © 2013 MD Conference Express®