Summary
A novel, thin-strut, metal stent with a mesh covering significantly improved the achievement of complete ST-segment resolution compared with standard stents after percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. This article reports the findings of the Safety and Efficacy Study of MGuard Stent after a Heart Attack [MASTER] trial.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Myocardial Infarction
A novel, thin-strut, metal stent with a mesh covering significantly improved the achievement of complete ST-segment resolution (STR) compared with standard stents after percutaneous coronary intervention (PCI) in patients with acute ST-segment elevation myocardial infarction (STEMI). Gregg W. Stone, MD, Cardiovascular Research Foundation, New York, New York, USA, reported the findings of the Safety and Efficacy Study of MGuard Stent after a Heart Attack [MASTER] trial, which were also published simultaneously in the Journal of the American College of Cardiology [Stone GW et al. 2012].
Dr. Stone described the stent used in the study as an embolic protection stent. The MGuard™ stent has a polyethylene terephthalate micronet sleeve covering that is designed to trap thrombi and friable atheromatous debris, thereby preventing distal embolization during PCI. PCI-induced distal embolization is thought to contribute to suboptimal myocardial perfusion after PCI, which is common and results in increased infarct size and mortality.
The MASTER trial enrolled 433 patients at 50 sites in 9 countries. All patients had acute STEMI, were seen within 12 hours of symptom onset, and were treated with emergent PCI. The patients were randomly assigned to treatment with the mesh-covered MGuard stent (n=217) or with a commercially available bare-metal or drug-eluting stent (n=216). The primary endpoint was the rate of complete STR, defined as a ≥70% reduction in the summed 12-lead extent of ST-segment elevation from the baseline electrocardiogram (ECG) to ECG done 60 to 90 minutes after PCI. Dr. Stone said complete STR is a strong surrogate for subsequent survival.
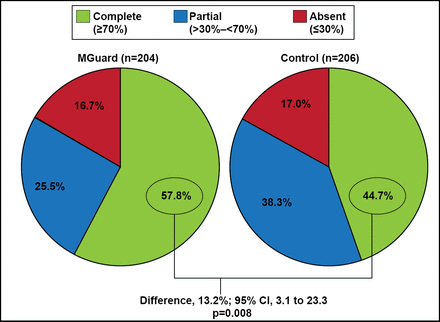
The trial met its endpoint with a significantly higher rate of complete STR for the MGuard stent compared with the standard stents (57.8% vs 44.7%; p=0.008; Figure 1). The MGuard stent was also associated with a significantly higher rate of TIMI-3 epicardial coronary flow compared with the standard stents (91.7% vs 82.9%; p=0.006). The rates of grade 2 or 3 myocardial blush were similar for the 2 groups (83.9% vs 84.7%; p=0.81).
Primary Endpoint: Complete ST-Segment Resolution.
Reproduced with permission from GW Stone, MD.
Dr. Stone reported clinical events at 30 days and acknowledged that the trial was underpowered for these events. The rate of major adverse cardiovascular events was similar for the MGuard stent and standard stent(1.8% vs 2.3%; p=0.75). Similarly, no significant difference in mortality was observed between the MGuard group (n=0) versus the standard group (n=4; p=0.06), but mortality trended in favor of the MGuard consistent with the STR findings.
Long-term clinical and angiographic follow-up of the patients in the trial is ongoing. A larger randomized trial is needed to determine whether the use of an embolic protection stent results in reduced infarct size and improved clinical outcomes.
- © 2012 MD Conference Express®