Summary
This article chronicles the history and progress of aortic valve replacement (AVR). The development of AVR surgery gave patients the opportunity to live longer, feel better, and benefit from improved left ventricular function [Bonow RO et al. Circulation 2008]. However, clinical trials in the 2000s revealed that >30% of patients with severe symptomatic aortic stenosis were untreated because they were elderly, had comorbidities with high operative risk, or refused surgery [Iung B et al. Eur Heart J 2005; Charlson E et al. J Heart Valve Dis 2006; Bach DS et al. Circ Cardiovascular Qual Outcomes 2009].
- Interventional Techniques & Devices
- Valvular Disease
Martin B. Leon, MD, NewYork-Presbyterian Hospital/Columbia University Medical Center, New York, New York, USA, chronicled the history and progress of aortic valve replacement (AVR). In 1968, Ross and Braunwald described the natural history of aortic stenosis (AS) as beginning with an asymptomatic latent period during which increasing obstruction and myocardial pressure overload occur. After onset of symptoms, survival is 50% at 2 years and 20% at 5 years. The development of AVR surgery gave patients the opportunity to live longer, feel better, and benefit from improved left ventricular function [Bonow RO et al. Circulation 2008]. However, clinical trials in the 2000s revealed that >30% of patients with severe symptomatic AS were untreated because they were elderly, had comorbidities with high operative risk, or refused surgery [Iung B et al. Eur Heart J 2005; Charlson E et al. J Heart Valve Dis 2006; Bach DS et al. Circ Cardiovascular Qual Outcomes 2009].
Development of Percutaneous Aortic Valve Replacement
The earliest version of the Andersen stent-valve was developed in 1989. In 1990, Alain Cribier, MD, began working on percutaneous valve technologies, ultimately developing valves from bovine and equine pericardium with a stainless steel stent. Transcatheter aortic valve replacement (TAVR) was first studied in sheep and cadavers in the early 2000s. Critics objected that TAVR would result in strokes, aortic rupture, coronary occlusion, mitral valve injury, and a host of other complications. In 2002, Dr. Cribier successfully performed the first human percutaneous transcatheter implantation of an aortic valve prosthesis for AS [Cribier A et al. Circulation 2002]. He used an antegrade approach, which is difficult to perform and can cause many complications, including cardiac arrest in up to 70% of patients. John Webb, MD, helped innovate the retrograde approach, and in 2004 Dr. Cribier and colleagues developed the transapical approach. TAVR development progressed from 2002 to 2012 with characterization of high surgical-risk patients, development of the heart team concept, and improved technology and procedural technique.
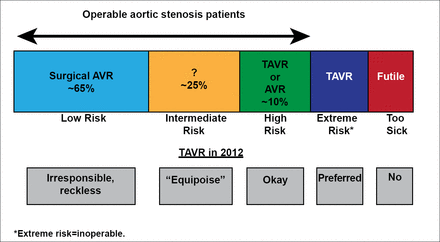
Patients with AS are categorized according to their level of surgical risk. Patients who are too sick for TAVR are referred to as “futile.” TAVR is preferred in patients at extreme risk for surgery and acceptable for patients at high surgical risk. TAVR is not appropriate for patients at low risk, but the suitability of TAVR for intermediate-risk patients is unclear (Figure 1).
TAVR Risk Categories.
AVR=aortic valve replacement; TAVR=transcatheter aortic valve replacement.
The heart team is crucial for patient selection and performance of TAVR, including specialists with skills in cardiac surgery, catheterization, and imaging. Multimodality imaging with angiography, echocardiography, and computed tomography angiography are needed for screening, guidance, and follow-up.
Progress in Clinical Research
Early research was problematic due to lack of standardization in patient risk profiles, endpoint definitions, devices, and procedural techniques. There were no core laboratories or adjudication of clinical events. Additionally, observational registry studies were underpowered and patient follow-up was inadequate. In response to these issues, the Valve Academic Research Consortium (VARC) developed standardized endpoint definitions for TAVR clinical trials [Leon B et al. J Am Coll Cardiol 2011; Eur Heart J 2011]. The VARC-2 update further expanded and refined clinical research processes [Kappetein AP et al. J Am Coll Cardiol 2012].
At present, more than 50,000 patients have undergone TAVR in over 500 interventional centers outside the United States. The Edwards Clinical Research Program has enrolled more than 11,500 patients across several clinical trials and valves have been implanted in more than 25,000 patients worldwide. CoreValve has an expanding evidence base with registry and randomized trials on long-term performance and quality of life, procedural best practices, and evaluation of performance in expanded populations. CoreValve's pivotal US trial [NCT01240902] in extreme-risk and high-risk patients is recruiting participants. Results of the German Aortic Valve Registry [GARY] study of 13,860 patients treated with surgery or TAVR were reported at the 2012 European Society of Cardiology (ESC) annual meeting [Hamm C et al. ESC 2012]. The TVT US National Registry is a new comprehensive, prospective, observational database that includes all patients treated in commercial approval trials with follow-up at 30 days and 1 year.
PARTNER Trial
The Placement of Aortic Transcatheter Valve Trial [PARTNER; NCT00530894] trial consisted of two individually powered parallel studies in inoperable (n=358) and high-risk (n=699) patients [Leon MB et al. N Engl J Med 2010; Smith CR et al. N Engl J Med 2011]. Inoperable patients were randomized to transfemoral TAVR or standard therapy. High-risk patients were randomized to a transfemoral or transapical approach, and each of those groups was randomized to TAVR or surgical AVR. The PARTNER Heart Valve Team included four surgeons and four interventionists.
Among inoperable patients, the primary endpoint, all-cause mortality, at 2 years was 30.7% in the TAVR group versus 50.7% in the standard therapy group (HR, 0.55; 95% CI, 0.40 to 0.74; p<0.001), demonstrating that TAVR is standard of care for inoperable patients [Leon MB et al. N Engl J Med 2010]. In the high-risk cohort, all-cause mortality was 24.2% with TAVR versus 26.8% with surgical AVR (HR, 0.93; 95% CI, 0.71 to 1.22; p=0.62), demonstrating TAVR to be a viable alternative to surgical replacement in appropriately selected high-risk patients with AS [Smith CR et al. N Engl J Med 2011].
A time-adjusted covariate analysis in the high-risk group showed that stroke, major bleeding, and major vascular complications were important predictors of mortality. At 30 days, neurologic events (stroke or trascient ischemic attack) were more common with TAVR (5.5%) versus surgical AVR (2.4%; p=0.04) and this difference persisted through the follow-up period (12 months, 8.3% vs 4.3%; p=0.04; 24 months, 11.2% vs 6.5%; p=0.05). Paravalvular regurgitation was associated with increased late mortality in TAVR patients (p<0.001) [Kodali SK et al. N Engl J Med 2012]. According to Dr. Leon, new techniques, better patient selection, better valve sizing, and better devices will reduce this problem in the future.
Current and Future State of TAVR
Average TAVR penetration across the 11 European Union Countries increased from ∼5% in 2009 to ∼13% in 2010 to ∼25% in 2011 [Piazza N et al. PCR London Valves 2012]. The US Food and Drug Administration (FDA) approved TAVR for inoperable patients in November 2011 and for high surgical risk patients using both the transfemoral and transapical approaches in October 2012 [FDA Device Approvals 2011; 2012].
Future technologies will include new TAVR systems, access and closure strategies, cerebral embolic protection devices, and advanced imaging modalities. New clinical indications and trials will include valve-in-valve for bio-prosthetic aortic and mitral valve failure and intermediate risk AS patients.
The use of TAVR has grown dramatically since 2002, but it remains a somewhat less predictable procedure than open AVR and is associated with several complications. Continued efforts to reduce these complications and refine patient selection are needed. Broader issues including economic constraints and inadequate physician training may limit TAVR penetration in the United States. Looking to the future, new technology advances promise
- © 2012 MD Conference Express®