Summary
Patients with locally advanced unresectable or metastatic soft tissue sarcoma have poor outcomes. Palliative chemotherapy is standard treatment for these patients, but it generally results in considerable toxicity. Previous studies have compared single-agent doxorubicin with doxorubicin plus ifosfamide, but the optimal doses remain unclear. This article discusses the European Organisation for Research and Treatment of Cancer 62012 Phase 3 study [NCT00061984] in patients with advanced soft tissue sarcoma.
- Soft Tissue Cancers
- Oncology Clinical Trials
Patients with locally advanced unresectable or metastatic soft tissue sarcoma have poor outcomes. Palliative chemotherapy is standard treatment for these patients, but it generally results in considerable toxicity. Previous studies have compared single-agent doxorubicin with doxorubicin plus ifosfamide, but the optimal doses remain unclear. The European Organisation for Research and Treatment of Cancer (EORTC) 62012 Phase 3 study [NCT00061984] presented by Winette van der Graaf, MD, PhD, Radboud University Nijmegen Medical Center, Nijmegen, Netherlands, compared single-agent doxorubicin (75 mg/m2) with doxorubicin (75 mg/m2) plus ifosfamide (7.5 g/m2) in patients with advanced soft tissue sarcoma (ASTS).
A total of 455 patients with high-grade ASTS were randomized to doxorubicin (n=228) versus doxorubicin plus ifosfamide plus pegfilgrastim (n=227). Patients were stratified according to age, performance status, liver metastases, and histologic grade. The most common histologic diagnoses were liposarcoma, leiomyosarcoma, and synovial sarcoma. The primary endpoint was overall survival (OS). The secondary endpoints were response, toxicity, and treatment-related mortality. The median follow-up was 56 months. An improvement in survival was defined as clinically significant if 1-year survival was at least 10% higher in the combination arm, corresponding with an HR <0.737. Allocated treatment was started by 217 patients in the doxorubicin arm and 215 patients in the combination arm.
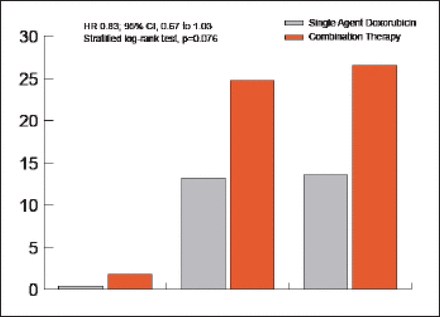
There was no significant difference in OS with doxorubicin versus combination treatment (HR, 0.83; 95.5% CI, 0.67 to 1.03; p=0.076; Figure 1). At 1year, OS was 51% in the doxorubicin arm versus 60% in the combination arm. The median OS was 12.8 months with single-agent doxorubicin versus 14.3 months with combination treatment. Progression-free survival (PFS) was significantly better in the combination arm compared with the doxorubicin arm (HR, 0.74; 95% CI, 0.60 to 0.90; p=0.003). The median PFS was 4.6 months with single-agent doxorubicin versus 7.4 months with doxorubicin plus ifosfamide. Patients treated with single-agent doxorubicin versus the combination had a complete response rate of 0.4% versus 1.8%, partial response rate of 13.2% versus 24.7%, and overall response rate of 13.6% versus 26.5%, respectively (Figure 1).
Response Rates with Single Agent Versus Combination Therapy.
Doxo=single-agent doxorubicin; Dxlf=doxorubicin plus ifosfamide.
Patients treated with doxorubicin plus ifosfamide versus single-agent doxorubicin had considerably higher rates of grade 3 or higher adverse events, including neutropenia (41.5% vs 37.2%), leukopenia (43.3% vs 17.9%), febrile neutropenia (45.9% vs 13.5%), anemia (34.9% vs 4.6%), and thrombocytopenia (33.5% vs 0.4%), respectively. A total of 121 patients in the doxorubicin arm versus 109 patients in the combination arm discontinued treatment because of progression of disease (PD) or death from PD (41.7% vs 20.7%); toxicity, including toxic death (2.6% vs 17.6%); patient refusal (1.8% vs 4.4%); death not related to malignant disease or toxicity (1.8% vs 0.4%); and other reasons (5.3% vs 4.8%), respectively.
The combination of doxorubicin and ifosfamide doubled the response rate and significantly improved PFS but did not significantly improve OS in patients with high-grade ASTS. Doxorubicin combined with ifosfamide was considerably more toxic than single-agent doxorubicin. The investigators concluded that single-agent doxorubicin should remain the standard treatment for this population.
- © 2012 MD Conference Express®