Summary
With the increasing the use of biomarkers in cancer treatment, the emphasis on selecting the right treatment for the right patient takes on added significance in early drug development. This article discusses the difficulties inherent in selecting patients for early drug-development studies.
- Affordability of Care
- Oncology Clinical Trials
- Molecular Imaging
- Oncology Genomics
- Reproductive Cancers
- Gastrointestinal Cancers
With the increasing the use of biomarkers in cancer treatment, the emphasis on selecting the right treatment for the right patient takes on added significance in early drug development. Steinar Aamdal, MD, PhD, Oslo University Hospital, Oslo, Norway, discussed the difficulties inherent in selecting patients for early drug-development studies.
Common strategies are to enroll “all comers” or to use screening to identify patients with specific biomarkers. The first strategy can lead to the enrollment of large numbers of patients who do not present with the target of interest. These patients may be placed at unnecessary risk and, if the drug response is low, their inclusion may result in approval of the drug being declined. Screening for patients with specific “driver mutations” is more selective but requires a large number of patients to be screened, and the effect of the drug in biomarker-negative population may not be detected. A third approach is adaptive randomization to targeted therapies based on relevant molecular biomarkers analyzed in fresh core-needle biopsy specimens.
The Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination [BATTLE] study showed that it was possible to conduct “personalized” lung cancer therapy by integrating real-time molecular laboratory findings in identifying patient populations for individualized treatment [Kim ES et al. Cancer Discov 2011]. Findings from this study may lead to the ability to match new therapeutics with diagnostics. Biomarker cutoff points should be selected with caution; however, too high may reduce discriminatory ability and too low may dilute the effect of the treatment in the positive-biomarker group. Challenges with this approach include obtaining the tissue for biomarker discovery (accessibility and the amount of available tumor tissue), the timeline for obtaining informed consent and prescreening before inclusion in the trial and intra- and intertumor heterogeneity.
Prof. Aamdal concluded by noting that when targeting driver mutations exist there are subsets of patients with mutations that still fail to respond or that response is often transient or short-lived, resistance can develop, and cures are rare. Thus, combination-targeted therapies and chemotherapy remain the backbone of cancer therapy for a number of solid cancers.
Jan Schellens, MD, PhD, The Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands, reviewed the benefits and safety features of some of the newer agents used to treat metastatic tumors.
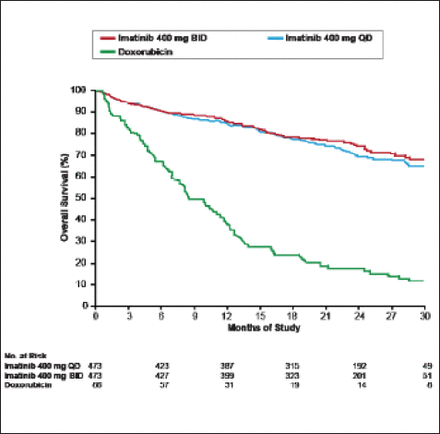
Following the results of a 2004 study [Verweij J et al. Lancet 2004] showing that imatinib resulted in significantly longer progression-free survival in patients with gastrointestinal stromal tumors, imatinib became the treatment of choice for most newly diagnosed patients (Figure 1). Recently, however, the appearance of imatinib resistance induced by mutations in the kinase domain of BCR-ABL has led to the search for second- and even third-line tyrosine kinase inhibitors, such as dasatinib and nilotinib, which has ushered in an era of more tailored or personalized therapy [Apperly JF et al. Lancet Oncol 2007; Guo T et al. Clin Cancer Res 2007]. Other targeted therapies have a selective mechanism of action and target on average one key protein, for example CD-20 (rituximab) or human epidermal growth factor receptor 2 (HER2; trastuzumab). Most have good or manageable safety records when used alone or in combination with other treatments. Selecting patients using a well-defined biomarker improves outcome.
GIST Survival: Imatinib Versus Doxorubicin.
GIST=gastrointestinal stromal tumor.
Reprinted from Verweij J et al. Progression-free Survival in Gastrointestinal Stromal Tumours with High-Dose Imatinib: Randomised Trial. The Lancet;364(9440):1127–34. Copyright 2004, with permission from Elsevier.
Ideally, new agents should selectively engage a unique, dominant target in cancer tissue; be easily combined with other agents to overcome tumor unresponsiveness; be devoid of normal tissue side effects; have acceptable absorption, good distribution to tumor tissue, known metabolism, and elimination; and be cost effective.
As more targeted therapies become available, additional emphasis is being placed on improved patient selection by molecular characterization of disease subtype. Molecular imaging may provide a noninvasive tool to quantify cellular targets for the entire disease burden, have the potential to serially evaluate the in vivo effects of a drug on the target, avoid unnecessary drug administration and toxicity, and might reduce treatment and clinical trial costs. Kristoff Muylle, MD, Institut Jules Bordet, Brussels, Belgium, presented data showing how molecular imaging can optimize targeted therapy in early drug development.
Results of the Neo-Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation [Neo-ALTTO] positron emission tomography (PET) substudy showed that [18F]-fluorodeoxyglucose PET/computed tomography (CT) was able to assess the early metabolic effects of anti-HER2 therapies and had a predictive value for pathological complete remission at the time of surgery in patients with early breast cancer [Gebhart G et al. J Nucl Med 2012]. Similarly, bone marrow activity concentration on immuno-PET/CT with 89Zr-rituximab was correlated to hematological toxicity in lymphoma patients. Both of these studies provide evidence that molecular imaging has the potential to evaluate and adapt therapeutic regimens and can aid in the development of patient-tailored image-guided therapy.
Ahmad Awada, MD, PhD, Institut Jules Bordet, Brussels, Belgium, outlined the challenges for targeted therapies in the future. Prof. Awada suggested that the basis of individualized oncology is matching the patient, tumor, and target in the context of vulnerability through whole disease progression; making sure the test-platform is reproducible and validated; and the drug or combination of drugs are selective or multitargeted.
Patient and tumor characteristics are important considerations in the selection of a targeted therapy. However, even though pharmacogenetics is emerging as a significant research field, it has not been shown to be useful in a clinical setting. Prof. Awada noted that there is no evidence to support CYP2D6 testing (involved in the tamoxifen metabolic pathway) in clinical practice.
Although there are many successful targeted therapies useful in molecularly selected patients, the difficult task of measuring the target/biomarker remains. There are enormous difficulties in ensuring reproducibility of measurement, selecting the right technology, and validating the results. With the recent discovery of de novo or acquired resistance mechanisms to targeted agents, the new task of finding agents to these resistant mutations has increased in significance.
Attention is also turning to the use of combination therapies to obtain maximum activity without overlapping toxicity and to overcome resistance by using non-cross-resistant drugs. Optimizing combination therapy can be best achieved by maximizing target and pathway inhibition (dual inhibition), and inhibiting parallel pathways, and feedback loops.
There is now no single methodology for the development of new targeted agents available. Individualizing and innovative drug development methodologies are key for success, taking into account the patient, the tumor, the target, and technology advances.
- © 2012 MD Conference Express®