Summary
This article discusses the characterization of non-small cell lung cancer (NSCLC), including its histology, biology, and molecular classification, and an integrative approach to its management. Lung cancer was first characterized by Rudolf Virchow in the 1800s as a tumor in the lungs that could be diagnosed by examining a pathological sample. While this definition is still in use today, lung cancer is, in fact, the result of multiple molecular alterations underlying the phenotypic changes observed in the cells.
- Respiratory Cancers
- Cancer
- Pulmonary Genomics
- Oncology Genomics
Jean-Charles Soria, MD, PhD, Institut de Cancérologie, Gustave Roussy, France, discussed the characterization of non-small cell lung cancer (NSCLC), including its histology, biology, and molecular classification, and an integrative approach to its management. Lung cancer was first characterized by Rudolf Virchow in the 1800s as a tumor in the lungs that could be diagnosed by examining a pathological sample. While this definition is still in use today, lung cancer is, in fact, the result of multiple molecular alterations underlying the phenotypic changes observed in the cells.
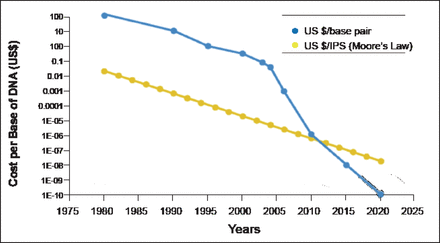
Prof. Soria explained the background of metastatic NSCLC. To define optimal therapy in daily clinical practice, NSCLC is primarily classified by histology as nonsquamous (adenocarcinoma and large cell carcinoma) or squamous cell carcinoma. He said that with appropriate treatment, the median overall survival for patients with these cancers is ∼12 months, and patients with anaplastic lymphoma kinase (ALK), reactive oxygen species (ROS), and epidermal growth factor receptor (EGFR) mutations have a median survival of ∼2 years. A better understanding of tumor biology is critical for selecting therapies that match a cancer's molecular features. As the cost of sequencing decreases (Figure 1) [MacConaill LE, Garraway LA. J Clin Oncol 2010], this goal is becoming more feasible.
DNA Sequencing Costs.
Reproduced from MacConaill and Garraway. Clinical implications of the Cancer Genome, Journal of Clinical Oncology 2010; 28(35):5219–5228. With permission from the American Society of Clinical Oncology.
Molecular Classification of NSCLC
Most sequencing efforts have focused on adenocarcinomas. Mutations have been found in 67% of adenocarcinomas; KRAS mutations (28%) are the most common, followed by EGFR (13%), serine/threonine kinase 11 (10%), and several others with an incidence of ≤2% [Planchard D et al. ELCC 2012]. Many of the identified mutations are not actionable, meaning no therapy has been developed to target them. For patients with EGFR and ALK mutations, erlotinib and crizotinib, respectively, have produced significant reductions in tumor burden [Rosell R et al. Lancet Oncol 2012; Kwak EL et al. N Engl J Med 2010].
Targeted therapies are not yet available for squamous cell lung cancer. The Cancer Genome Atlas recently published molecular profiling results showing that squamous cell lung cancer is characterized by a high rate of genomic alterations [Cancer Genome Atlas Research Network. Nature 2012]. According to Prof. Soria, the fibroblast growth factor receptor 1 (FGFR1) mutation is one potential therapeutic driver in this cancer. FGFR1 mutations are found in 10% of squamous cell lung cancers by comparative genomic hybridization and 22% by fluorescence in situ hybridization [Weiss J et al. Sci Transl Med 2010]. FGFR has been targeted by selective (BJG398, AZD 4547, and JNJ-42756493) and nonselective inhibitors (EOS3810 and dovitinib). Potential therapeutic targets and their frequencies in squamous cell lung cancer include FGFR1 (22%), DDR2 (4%), PIK3CA (33%), MET (6%), and BRAF (2%) [Perez-Moreno P et al. Clin Cancer Res 2012]. FGFR1/2, PIK3CA, and DDR2 inhibitor trials are ongoing. In the near future, patients with a lung nodule will not only undergo a tumor biopsy but also comprehensive molecular testing to determine the diagnosis, prognosis, chemotherapy sensitivity, and targeted therapy sensitivity, and to identify new targets.
Towards an Integrative Approach
Individual tumor heterogeneity presents a limitation to developing molecular targets [Meric-Bernstam F, Mills GB. Nat Rev Clin Oncol 2012]. Treatment with a drug targeted at a specific alteration will eliminate that particular clone; however, other clones will remain. Further, as a cancer progresses and metastasizes, additional biopsies will need to be performed to identify and target emerging gene alterations. Another issue pertains to the one third of patients without an identified alteration. For these patients, it is important to optimize the use of chemotherapy and radiotherapy, and develop a better understanding of DNA repair. These patients also present an opportunity to develop new immunomodulatory checkpoints and to define predictive biomarkers.
DNA repair pathways are crucial to cell survival. Normal cells have 6 interindependent DNA repair pathways; recent studies have shown that when 1 pathway fails, another takes over. These pathways include base excision repair, nucleotide excision repair, direct repair, mismatch repair, homologous recombination repair, and nonhomologous end-joining repair [Postel-Vinay S et al. Nat Rev Clin Oncol 2012].
In cancer cells, there is at least 1 repair pathway that is often defective [Shaheen M et al. Blood 2011; Postel-Vinay S et al. Nat Rev Clin Oncol 2012]. Adenocarcinomas and squamous cell cancers contain different levels of molecules involved in DNA repair. For example, adenocarcinomas express low levels of poly [ADP-ribose] polymerase 1 and breast cancer 1/2 compared with squamous cell cancers. This is also true for checkpoint kinase 1 expression. These differences can be exploited in the development of DNA repair modulators.
Immunohistochemistry has not been sufficient to identify the presence of a deficient DNA repair pathway in tumor specimens. Prof. Soria has been working on developing a functional DNA repair assay for use on fresh lung tumor specimens, which he believes is necessary for developing and optimizing therapeutics in this area.
Immunogenic modulation is another area with potential for therapeutic development in lung cancer. In addition to cytotoxic T-lymphocyte antigen 4, the programmed death-1 (PD-1) protein and its ligand, PD-L1, are potential targets for which antibodies are currently in development in at least six Phase 1 and 2 trials (Table 1).
Studies of Antibodies Targeting PD-1 and PD-L1 Pathways.
The PD-1 antibody BMS-936558 has demonstrated objective response rates of 33% (95% CI, 13% to 59%) in squamous cell cancer and 12.5% (95% CI, 5% to 24%) in NSCLC [Brahmer JR et al. N Engl J Med 2012]. Other recent evidence has shown a correlation between PD-L1 expression in pretreatment tumor biopsies with clinical outcomes in 16 of 42 patients, including those with NSCLC (n=10), melanoma (n=18), colorectal cancer (n=7), renal cell cancer (n=5), or castration-resistant prostate cancer (n=2) [Soliman HH et al. ASCO 2012. Abstract 2501].
Prof. Soria concluded that the future of patients with lung cancer depends on the ability to offer them a molecular portrait revealing the specific mutations and amplifications that can then be targeted with existing therapies, such as gefitinib, erlotinib, and afatinib (EGFR inhibitors), crizotinib (ALK and ROS inhibitor), trastuzumab (human epidermal growth factor receptor 2 inhibitor), and BJG398, AZD4547, and EOS3810 (FGFR1 inhibitors). For patients with no identified genetic alterations, treatment relies on standard chemotherapy and radiotherapy. DNA dysfunctionality analysis and new immune checkpoint identification should be pursued in these patients with the goal of entering them into appropriate clinical trials.
- © 2012 MD Conference Express®