Summary
The recognition that cervical cancer is caused by human papillomavirus (HPV) has opened new opportunities for preventing this devastating cancer. This article presents evidence for the feasibility of eliminating cervical cancer through combined screening and HPV vaccination.
- Viral Infections
- Reproductive Cancers
- Sexually Transmitted Diseases
- Screening & Prevention Reproductive Cancers
- Vaccinations
The recognition that cervical cancer is caused by human papillomavirus (HPV) has opened new opportunities for preventing this devastating cancer. Jack Cuzick, PhD, Saint Bartholomew's Medical School, London, United Kingdom, presented evidence for the feasibility of eliminating cervical cancer through combined screening and HPV vaccination.
HPV and Cytology Screening for Cervical Cancer
To detect cervical cancer, European and North American screening studies have suggested primary HPV screening as a better method than traditional cytological methods. In a large pooled analysis which included 60,000 women included, HPV screening was more sensitive than cytology (96.1% vs 53%) in detecting cervical intraepithelial neoplasia grade 2 or greater (CIN2+) but less specific (90.7% vs 96.3%) [Cuzick J et al. Int J Cancer 2006]. In a multinational cohort, the cumulative incidence of cervical intraepithelial neoplasia grade 3 or cancer (CIN3+) after 6 years was considerably lower among women negative for HPV at baseline than among women with negative cytology at baseline [Dillner J et al. BMJ 2008]. The data support the use of HPV testing as the primary screening test for cervical cancer.
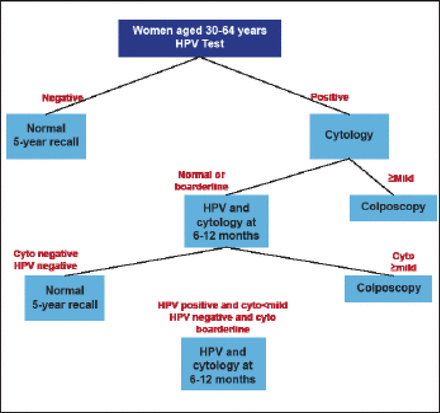
Figure 1 shows a proposed new screening algorithm beginning with HPV testing, followed by cytology screening for those with positive HPV results and colposcopy for patients with abnormal cytology. In the future, the algorithm may be modified and simplified by including HPV-16 typing and testing for p16 overexpression to reduce referrals for nonprogressive HPV infections.
Proposed New Screening Algorithm.
Cyto=cytology; HPV=human papillomavirus.
Reproduced with permission from J Cuzick, PhD.
HPV Vaccination
Primary prevention of cervical cancer is possible with HPV immunization of adolescents and young women. The Cervical Intraepithelial Neoplasm (CIN) in Women [FUTURE II; NCT00092534] trial of a quadrivalent vaccine against HPV-6/11/16/18 included a per-protocol susceptible population of 5305 women in the vaccine group and 5260 women in the placebo group [FUTURE II Study Group. N Engl J Med 2007]. At 36 months median follow-up, the vaccine efficacy for preventing the composite endpoint of CIN2–3, adenocarcinoma in situ, or HPV16/18-related cervical cancer was 98% (95.89% CI, 86 to 100). Nonavalent vaccines against HPV-16/18, HPV-6/11 (for prevention of genital warts), and 5 new oncogenic types (31, 33, 45, 52, and 58) are currently under development.
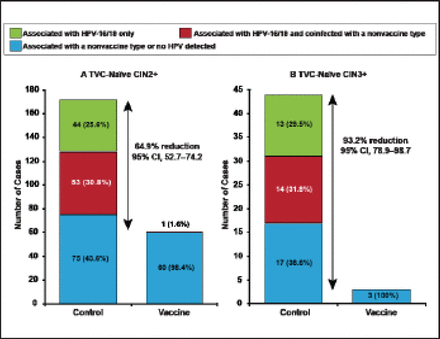
The HPV Vaccine Efficacy Trial Against Cervical Pre-cancer in Young Adults with GlaxoSmithKline (GSK) Biologicals HPV-16/18 [PATRICIA; NCT00122681] trial randomized 18,644 women to HPV-16/18 vaccine versus control [Lehtinen M et al. Lancet Oncol 2012]. After a mean follow-up of 44.2 months, vaccine efficacy against CIN3+ associated with HPV-16/18 was 100% (95% CI, 85.5 to 100) in the total vaccine cohort-naïve. Vaccine efficacy against all CIN3+ was 93.2% (95% CI, 78.9 to 98.7; Figure 2).
Vaccine Efficacy in the Total Vaccine Cohort-Naïve.
CIN2+=cervical intraepithelial neoplasia grade 2 or greater; CIN3+=CIN grade 3 or greater; TVC=total vaccine cohort.
Reprinted from Lehtinen M et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncology;13(1):89–99. Copyright 2012, with permission from Elsevier.
Issues associated with HPV vaccination include the need for 3 doses, cross-protection against other HPV types, durability of protection, the focus on adolescent girls, vaccination of boys, the need for vaccines not requiring cold storage, and lack of effectiveness after HPV infection. Vaccination coverage can be improved in the future by extending the age range for vaccination to children and adult women, and by alternative dose schedules (eg, 2 doses) [Kreimer AR et al. IPvC 2010]. Cervical cancer rates can be further reduced by screening older women to eliminate all current disease and vaccinating with polyvalent vaccines to prevent future disease. Next-generation HPV vaccines include polyvalent L1 virus-like particle (VLP) vaccines, L2 peptide vaccines, chimeric L1/L2 VLP vaccines, and combined prophylactic and therapeutic HPV vaccination [Kanda T and Kondo K. Hum Vaccin 2009; Stanley M. Curr Opin Infect Dis 2010].
Cervical cancer is the only cancer with a single, known cause: HPV. Vaccination can prevent infection but not eliminate it or impact subsequent cancer once it occurs. Cytology screening can identify treatable precursor lesions in women who are found to have HPV infection. Prof. Cuzick concluded that combined screening and vaccination in women aged 25 through 50 years offers the best chance of eliminating cervical cancer.
- © 2012 MD Conference Express®