Summary
This article reviews the main reasons why drugs fail in triple-negative breast cancer (TNBC) and targeted agents for the treatment of TNBC, as well as discusses ways that molecular triaging can assist in the selection of patients for clinical trials, the role of new cytotoxic agents in TNBC, and angiogenic vascular cells, infiltrating immune cells, and cancer-associated fibroblasts, and adipocytes as host components
- Oncology Genomics
- Breast Cancer
Fabrice André, MD, PhD, Institut Gustave Roussy, Villejuif, France, reviewed the main reasons why drugs fail in triple-negative breast cancer (TNBC).
Firstly, the options for the treatment of TNBC, which represents 15% to 20% of all breast cancers, are currently limited—indicating an important unmet need; and an intensified and possibly too rapid effort is being made to develop new approaches. An example is the poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitor iniparib, which demonstrated efficacy in a Phase 2 randomized trial [O'Shaughnessy J et al. New Engl J Med 2011]; however, a subsequent Phase 3 registration trial failed to confirm the initial results [O'Shaughnessy J. ASCO 2011 Abstract 1007].
Secondly, TNBC is a highly heterogeneous disease with a large proportion of patients having a unique disease on the genomic level. This results in very small individual Phase 2 trials that cannot consistently generate robust data. Larger Phase 2 trials should be conducted by developing consortiums of referral centers focusing on specific patient categories.
Thirdly, there is a need to evaluate TNBC therapies in homogeneous populations that are defined by biomarkers. One example is a trial currently underway to identify a biomarker for bevacizumab efficacy [Miles DW et al. SABCS 2010].
Fourthly, chemotherapy remains the backbone treatment in TNBC, and this has to be considered in clinical trial design. Patients with TNBC in early studies of sunitinib monotherapy had positive outcomes [Burstein HJ et al. J Clin Oncol 2008]; however, a Phase 3 trial comparing the efficacy of sunitinib plus docetaxel versus docetaxel alone in patients with advanced breast cancer was negative [Bergh J et al. J Clin Oncol 2011]. Importantly, this registration trial evaluated sunitinib plus suboptimal dose chemotherapy versus full-dose chemotherapy alone; it did not evaluate sunitinib plus full-dose chemotherapy. If allowed by side effects, targeted agents should be evaluated in combination with full-dose chemotherapy.
Other issues important for drug development include choice of drug combinations (different combinations can give different results), appropriate outcome measurements (measurement of overall survival is important because of poor prognosis), and appropriate identification of efficacy signals in patients with resistance to conventional therapy.
Lajos Pusztai, MD, DPhil, Yale School of Medicine, New Haven, Connecticut, USA, discussed ways that molecular triaging can assist in the selection of patients for clinical trials. The first fundamental concept of triaging is that a hypothesis exists regarding which molecular marker might identify a patient population potentially susceptible to a particular drug. Currently identified marker abnormalities are rare due to the heterogeneity of the disease, but as a group, these markers can be found in >50% of patients. Therefore, the most optimal strategy is to look for many different markers and then assign the patient to the particular study that may work for that particular cancer. A second fundamental concept is that there must be a portfolio of clinical trials that match the portfolio of available assays and abnormal markers. This strategy is particularly appealing for TNBC because of the lack of recent clinical therapeutic advances and because a plethora of potential targets and good ideas about how to approach them exists. Good ideas come from 2 sources: preclinical model systems and novel targets in identified in human genomic studies.
However, not all biomarkers are of equal potential value in predicting outcome or therapeutic response. Past evaluations of the predictive or prognostic role of the immune system have been contradictory. For example, prognostic value can be lost without first separating cancer by hormone receptor status. One German study revealed that immune cell presence was prognostic in estrogen receptor (ER)-positive highly proliferative and ER-negative cancers only [Bianchini G et al. J Clin Oncol 2010].
There are dramatic expression differences in kinases, and these differences exist in 2 forms: those that are immediately targetable and directly testable, and those that are potential new drug targets. While individual mutations in breast cancer are very rare, there are proven drugs that may target particular mutations. These mutations may be grouped by pathway (eg, mammalian target of rapamycin pathway). There is a targetable abnormality in the majority of patients with TNBC. Molecular triaging helps predict how this relates to therapy.
Dr. Pusztai emphasized Prof. André's point about the necessity to form consortia to account for the molecular diversity of TNBC. Molecular markers are increasingly used in clinical trials as patient selection criteria or as an enrichment strategy. However, enrichment does not power a study to reach a definitive conclusion. In order to state valid conclusions, the number of patients should be calculated a priori. Because no single individual will have access to all clinical trials, investigators should team up and create a central database so patients can have access to an appropriate trial.
Nicholas Turner, MD, Institute of Cancer Research, London, United Kingdom, reviewed targeted agents for the treatment of TNBC. As noted by the previous presenters, TNBC can be differentiated into several subtypes according to molecular profiling [Lehmann BD et al. J Clin Invest 2011]. These include basal-like, immunomodulatory, mesenchymal-like, and luminal-androgen receptor.
Common TNBC genetic mutations include tumor protein 53 (TP53) and breast cancer 1 and breast cancer 2 (BRCA1/2). BRCA1/2 mutations are potentially targetable by PARP inhibitors [Jonkers J et al. SABCS 2011]. Historically, targeting TP53 inactivation has been challenging, although preclinical data suggest that combinations of DNA damaging chemotherapy with checkpoint kinase 1 or WEE1 inhibitors have the potential to target TP53 deficiency [Yu X et al. Cancer Cell 2012].
Javier Cortes, MD, Vall d'Hebron University Hospital and Institute of Oncology, Barcelona, Spain, discussed the role of new cytotoxic agents in TNBC. Currently, the median survival time from distant recurrence to death is only 9 months in patients with TNBC [Dent P et al. Clinical Cancer Res 2007]. As previously noted, no specific therapeutic regimen guidelines currently exist, and there are few data on which to base therapeutic decisions. Recurrence rates for current first-line therapies are high.
Exploratory, Phase 2 data of nanoparticle albumin-bound paclitaxel [Blum JL et al. Clin Breast Cancer 2007] and ixabepilone monotherapy [Perez EA et al. J Clin Oncol 2007] in TNBC reveal response rates similar to that of the overall breast cancer population. A Phase 3 study of ixabepilone plus capecitabine versus capecitabine monotherapy showed improved efficacy for the combination [Sparano JA et al. J Clin Oncol 2007]. Eribulin (E7389), a novel tubulin targeted agent [Jordan MA et al. Mol Cancer Ther 2005], showed an objective response rate (all partial responses [PRs]) of 11.5% and a clinical benefit rate (PR plus stable disease ≥6 months) of 17.2% (95% CI, 10.0 to 26.8) in patients with metastatic breast cancer who were previously treated with an anthracycline and a taxane, including patients with TNBC [Vahdat LJ et al. J Clin Oncol 2009. A Phase 2 trial of eribulin mesylate showed similar benefit for patients with TNBC and patients with other breast cancer subtypes [Cortes J et al. Lancet 2011]. Etirinotecan pegol, a tumor-targeted topoisomerase I inhibitor, was effective in approximately 40% of patients with TNBC (n=30) in a Phase 2 trial [Awada A et al. IMPAKT 2012].
Guisseppe Curigliano, MD, PhD, Istituto Europeo di Oncologia, Milan, Italy, focused on angiogenic vascular cells, infiltrating immune cells, and cancer-associated fibroblasts, and adipocytes as host components.
Angiogenic vascular cells sustain proliferative signaling through angiogenic switch and increased rates of cancer cell proliferation; additionally, they resist cell death via abnormal vasculature, which fuels tumor progression and treatment resistance. Lastly, they activate invasion and metastasis via tumor vasculature that is hyperstimulated by vascular endothelial growth factor reductions in pericyte coverage. Examples of therapeutic targets include bevacizumab [Thomssen C et al. Oncology 2012] and sunitinib [Curigliano G et al. Submitted 2012].
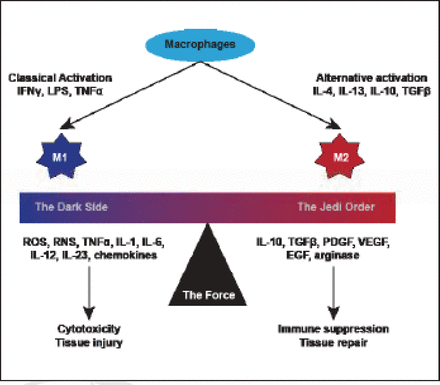
Infiltrating immune cells sustain proliferative signaling via inflammation and cancer, and release of mitogenic growth mediators. Furthermore, they resist cell death via tumor-associated macrophages that promote survival in metastatic breast cancer cells, and they activate invasion and metastasis via colony stimulating factor 1 signaling (Figure 1).
Macrophage Polarization.
Reproduced with permission from G Curigliano, MD, PhD.
The tumor microenvironment sustains proliferative signaling via fibroblasts proximal to tumors that are “educated” and differentiated into other cells. They resist cell death via cancer-associated fibroblasts that can orchestrate functional attributes associated with epithelial-to-mesenchymal transition.
A diverse set of potentially targetable oncogenic mutations and amplifications occur in TNBC that are likely important therapeutic targets for individual cancers; however, these present a substantial challenge to drug development due to their individual rarity. Combination therapy will be critical to treating this disease. Identification of biomarkers for targeted treatment is a necessary step to development and testing of targeted therapies. The challenge is how to integrate multiple molecular abnormalities into a rational proposal for creating an efficient drug cocktail that matches the tumor abnormalities.
- © 2012 MD Conference Express®