Summary
The level of disease (single or multilevel), the absolute number of involved lymph nodes, the possible presence of bulky disease, and downstaging after induction can all have an impact on outcome and survival of non-small cell lung cancer (NSCLC). This article discusses factors that should be considered prior to surgery for stage III NSCLC, optimal radiotherapy combined with chemotherapy for stage III NSCLC, as well as a review of some of the data and ongoing trials for targeted agents in stage I to III NSCLC.
- Adjuvant/Neoadjuvant Therapy
- Cancer
- Respiratory Cancers
- Radiology
The heterogeneity of disease in the subgroups of stage III non-small cell lung cancer (NSCLC) must be considered when selecting treatments for individual patients and patients for participation in clinical trials. The level of disease (single or multilevel), the absolute number of involved lymph nodes, the possible presence of bulky disease, and downstaging after induction can all have an impact on outcome and survival. Georgios Stamatis, MD, Ruhrlandklini/University Essen, Essen, Germany, discussed factors that should be considered prior to surgery for stage III NSCLC.
Results from a study in patients with resected stages II and IIIa NSCLC indicated no difference in survival among patients with left upper lobe NSCLC and metastases to single-level N2 lymph nodes and patients with N1 disease; however, the presence of isolate N2 skip metastases was associated with improved survival when compared with patients with both N1 and N2 disease. The authors suggested that these results may be useful in informing clinical trial development, treatment strategies, and revisions of the tumor node metastasis staging system [Keller SM et al. J Thorac Cardiovasc Surg 2004]. In another study that compared the prognostic value of the number of metastatic lymph nodes in resected NSCLC with the currently used nodal stage classification, the number of lymph nodes was found to be a better prognostic indicator [Wei S et al. J Thorac Oncol 2011]. Betticher et al. [J Clin Oncol 2003] found that in patients with locally advanced NSCLC with tumor resection, downstaging to N0–1 at surgery was prognostic and significantly prolonged event-free survival and overall survival (OS; p=0.0001).
Clinical studies have shown that concurrent chemoradiotherapy is superior to sequential chemo-radiation in terms of OS. Dirk De Ruysscher, MD, PhD, Leuven Cancer Institute, Leuven, Belgium, discussed optimal radiotherapy combined with chemotherapy for stage III NSCLC.
Results of a recent meta-analysis that directly compared the 2 approaches in NSCLC patients support the earlier individual studies (HR, 0.84; 95% CI, 0.74 to 0.95; p=0.004 in favor of concurrent therapy) and also indicated that the increased OS was mostly due to improvements in locoregional control [Aupérin A et al. J Clin Oncol 2010]. Although increasing locoregional control may be one way to improve OS, the rate of local progression was still 30% to 40% and there was no difference in the incidence of distant metastases indicating that additional methods to optimize treatment are needed.
Most clinical studies are based on the standard concurrent chemoradiotherapy approach for stage III (T4, N2/3) NSCLC: a radiotherapy schedule of 60 to 66 Gy in 2 Gy/day fractions 5 times per week with concurrent cisplatin-etoposide, cisplatin-vinorelbine, or carboplatin-paclitaxel (CP). However, some small trials have shown a survival advantage with high-dose radiotherapy. To test this hypothesis, the Radiation Therapy Oncology Group (RTOG) conducted a randomized Phase 3 trial [RTOG 0617; NCT00533949]. Patients with NSCLC that had spread to the lymph nodes were treated with weekly CP and either 60 or 74 Gy in 2 Gy QD fractions, either with or without cetuximab, followed by 2 cycles of consolidation CP ± cetuximab. At the 11-month interim analysis, the high Gy-dose arm showed worse OS compared with patients in the low Gy-dose arm (HR, 1.45; 95% CI, 1.02 to 2.05; p=0.02). Median survival was 21.7 months with 60 Gy versus 20.7 months with 74 Gy [Bradley JD et al. ASTRO 2011. Abstract LB2]. These unexpected results are still being investigated. Possible explanations include the preliminary nature of the results, the possibility of an interaction between 74 Gy and cetuximab, the specific effects of cetuximab (no subanalysis has been released for this group), the potential added toxicity of adjuvant chemotherapy and 74-Gy radiotherapy, and/or other technical factors. This does not mean that dose intensification is not being pursued. There are trials looking at biological dose escalation (standard total doses given over a shorter overall time) and individualization of treatment using physical and biological approaches to determine which patients would benefit from accelerated or high-dose treatments. For standard practice, current protocols and guidelines should not be changed but emphasis on quality assessment and, if needed, adjustment of the entire diagnostic, treatment and supportive care process should be the main goal, concluded Prof. De Ruysscher.
Targeted agents are promising for selected patients with resectable stage I to III NSCLC, but it is clear at present that this is not a standard of care and patients for whom such therapy is being considered should be enrolled in clinical trials. Oliver Gautschi, MD, Luzerner Kantonsspital, Lucerne, Switzerland, reviewed some of the data and ongoing trials for targeted agents in stage I to III NSCLC.
Although improving, there is clearly much that can still be done to increase the OS in patients with early NSCLC. Tumor mutations can affect prognosis and may be useful to help guide the administration of specific types of adjuvant therapy [Marks JL et al. J Thorac Oncol 2008; Tsao MS et al. J Thorac Oncol 2011]. Treatment with the EGFR inhibitor gefitinib was associated with a significant reduction in survival when it was evaluated in a maintenance setting in molecularly unselected patients with stage III NSCLC who were post radiation [SWOG 0023; Kelly K et al. J Clin Oncol 2008]. In the NCIC CTG BR.19 trial, OS with adjuvant gefitinib after complete resection of NSCLC was not different from placebo in the overall population, and KRAS and EGFR mutations were neither prognostic nor predictive of benefit from gefitinib by subgroup analysis [Goss GD et al. ASCO 2010. LBA7005].
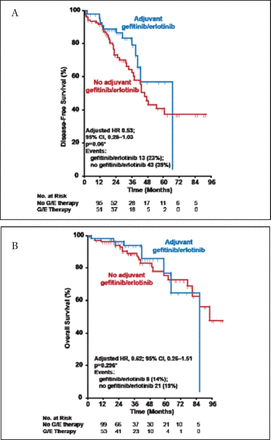
In a retrospective cohort study that evaluated adjuvant EGFR tyrosine kinase inhibitors (TKIs) in patients with stage IV lung adenocarcinoma and EGFR mutation at the MSKCC, adjuvant TKI therapy was associated with a trend toward improvement in 2-year disease-free survival (DFS HR, 0.53; 95% CI, 0.28 to 1.03; p=0.06; Figure 1A) but no difference in OS (Figure 1B) [Janjigian YY et al. J Thorac Oncol 2011]. In a follow-up of the same cohort, the investigators concluded that recurrence of EGFR-mutant lung cancer after stopping adjuvant TKI should not preclude a trial of TKI retreatment [Oxnard GR et al. J Clin Cancer 2011] This was recently confirmed by the results of the Surgery for Early Lung Cancer with Preoperative Erlotinib: A Clinical Phase II Trial [SELECT; NCT00462995] evaluating the safety and efficacy of adjuvant erlotinib in EGFR mutation-positive NSCLC, which showed a 2-year DFS of 94% (95% CI, 0.80 to 0.99) [Neal JW et al. ASCO 2010. Abstract 7010] and successful reintroduction of the TKI after relapse. Several other trials evaluating the use of TKIs in the adjuvant setting are ongoing, including RADIANT [NCT00373425] and ADJUVANT [NCT01405079]. In the neoadjuvant setting, so called “window-of-opportunity” trials are also ongoing, and are important and suitable to explore and validate new drugs and predictive markers. Presently, targeted therapies in the neoadjuvant setting remain experimental, but they are promising and may replace neoadjuvant chemotherapy in selected patients in the near future.
Memorial Sloan-Kettering Cancer Center Cohort: DFS and OS.
Janjigian YY et al. Impact on Disease-Free Survival of Adjuvant Erlotinib or Gefitinib in Patients with Resected Lung Adenocarcinomas that Harbor EGFR Mutations. J Thorac Oncol 2011;6(3):569–575.
- © 2012 MD Conference Express®