Summary
This article discusses the concept of window-of-opportunity studies in relation to squamous cell cancers of the head and neck. Also discussed are predictors of sensitivity and resistance mechanisms, a review of poly (adenosine diphosphate-ribose) polymerase inhibitors and strategies in chemoradiation.
- Radiology
- Radiation Therapy
- Head & Neck Cancers
Jean-Pascal Machiels, MD, PhD, Université Catholique de Louvain, Brussels, Belgium, discussed the concept of window-of-opportunity studies in relation to squamous cell cancers of the head and neck (SCCHN).
Window-of-opportunity studies involve the testing of therapeutic agents during the preoperative setting (ie, between diagnosis and surgery). This study design is increasingly popular because it allows the collection of biological data pre- and post-treatment; it is frequently used in breast cancer. SCCHN represents an attractive disease for window-of-opportunity studies because tumors are generally easily accessible to iterative biopsies. Considerations for these studies include the assurance of safety in treatment, avoidance of treatment delays [Primdahl H et al. Acta Oncol 2006; Jensen AR Radiother Oncol 2007], prospective definition of biopsies and imaging details, and standardization of drug schedule, dose, and duration of treatment.
In terms of head and neck cancer, it is known that the anti-epidermal growth factor receptor (EGFR) antibody cetuximab improves overall survival in combination with radiotherapy (RT) or chemotherapy [Bonner JA et al. N Engl J Med 2006; Vermorken JB et al. N Engl J Med 2008]. However, only a minority of patients benefit from this treatment [Machiels JP et al. Lancet Oncol 2011]; therefore, it is important to better understand the molecular biology of this compound. In addition, surgery-related release of epidermal growth factor-like factors might promote cell proliferation leading to tumor recurrence [Licitra L et al. Ann Oncol 2011].
Prof. Machiels and colleagues conducted a window-of-opportunity study with cetuximab in SCCHN to determine the surgical safety of preoperative cetuximab administration [Schmitz S. Ann Oncol 2012 (suppl 9)]. Cetuximab was given for 2 weeks prior to surgery; 2-[fluorine-18]-fluoro-2-deoxy-D glucose positron emission tomography (18FDG-PET), computed tomography scan or magnetic resonance imaging, tumor biopsies, and plasma collection were performed before and after treatment. Eighty percent of patients in the cetuximab arm demonstrated a partial response via 18FDG-PET. The safety and feasibility of this approach was demonstrated.
Amanda Psyrri, MD, National and Kapodistrian University of Athens, Athens, Greece, discussed predictors of sensitivity and resistance mechanisms. A research priority is to better elucidate the underlying causes of intrinsic or acquired resistance to EGFR-targeted agents so that novel treatment strategies can be developed to potentiate the impact of EGFR inhibitors. Unfortunately, these mechanisms of resistance are largely unknown. Potential mechanisms include the following: 1) constitutive up-regulation of downstream targets of EGFR, 2) compensatory up-regulation of redundant receptor tyrosine kinases that signal through common effectors, 3) ligand-independent signaling, 4) transcriptional regulatory mechanisms that control EGFR expression, 5) inhibition of the ubiquitin-mediated degradation of EGFR, and 6) epithelial to mesenchymal transition.
Currently, no biomarker is predictive for response to cetuximab in treatment of SCCHN. Several biomarkers have been proposed as predictive for response to cetuximab in small cohorts, but none have been validated. For example, Wheeler et al. [Clin Can Res 2012] demonstrated that high EGFR protein level by immunohistochemistry was associated with reduced survival (p=0.06) in a small cetuximab-treated cohort.
Kevin Harrington, MD, Royal Marsden Hospital, London, United Kingdom, presented a review of poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) and strategies in chemoradiation.
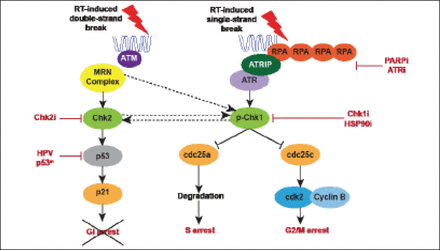
Platinum-based chemoradiotherapy (CRT) is the gold standard for treatment of stage III/IV SCCHN. Most SCCHN have defects in the DNA damage response, such as in p53 pathway signaling; these occur either through mutation or post-translational silencing by viral oncoproteins. Therefore, head and neck cancers are reliant on the G2/M cell cycle checkpoint, which represents a potential point of attack for strategies combining RT and novel targeted radiosensitizers (Figure 1).
Targeting G2/M Checkpoint Control in Head and Neck Cancer.
RT=radiotherapy.
Reproduced with permission from KJ Harrington, MD.
(PARPi) are potent radiosensitizers that have been evaluated in combination with RT or CRT. Research by Dungey et al. demonstrates an S-phase dependence; the more cells that are cycling, the greater sensitivity to PARPi and response to radiation [Dungey FA et al. In J Radiat Oncol Biol Phys 2008]. At least 3 studies of PARPi in combination with RT are currently underway.
Checkpoint kinase 1 (Chk1) inhibition in combination with RT in vitro and in vivo is being conducted in the laboratory of Dr. Harrington [Borst GR et al. Int J Radiat Oncol Biol Phys 2012]. Colony and caspase activity assays in cell lines with nonfunctional p53 revealed significant radiosensitization by Chk1 inhibition, in contrast to the effect on cell lines with functional p53. Abrogation of RT-induced G2 phase arrest via Chk1 inhibition was compensated by an increase in G1 phase in population for cell lines with functional p53. Significant delays in tumor growth were seen in vivo, as well as increased survival in animal models.
Heat shock protein 90 (HSP90) inhibition maintains the stability and activity of several proteins key to cell cycle arrest, DNA damage repair, and apoptosis linked to cellular response to radiation. HSP90 is an important cellular chaperone; proteins require this chaperoning and subsequent folding to become functional. HSP90 inhibitors can prevent this chaperoning and folding of the substrate, and the substrate will subsequently be degraded. NVP-AUY922, an HSP90 inhibitor, potently radiosensitized a selection of cell lines corresponding to depletion of radioresistance markers at equivalent concentrations [Zaidi S et al. PLoS One 2012]. Delayed tumor growth and increased survival were seen in vivo. NVP-AUY922 in combination with RT exhibits multitarget interference in cell cycle progression and DNA damage repair.
In the context of low-risk disease, platinum may be replaced with targeted agents such as this. In contrast, combination therapy is likely necessary for patients with high-risk disease. The possibility exists for the combination of platinum-based agents with targeted agents.
The preoperative setting is an attractive mechanism for investigation of new therapeutic agents. Endpoints for window-of-opportunity studies should be defined a priori. Window-of-opportunity studies are both safe and feasible in SCCHN. Head and neck cancers are an excellent model for testing novel drug therapies in combination with RT/CRT.
Opportunities exist for exploitation of synthetic lethality with RT. Targeting checkpoint control/DNA damage response at G2/M is a rational strategy. Finally, patient stratification is key to future trial design for both escalation and de-escalation. Strategies to optimize EGFR-targeted therapy in head and neck cancer include selection of patients most likely to derive benefit, as well as the use of combination strategies to override resistance.
- © 2012 MD Conference Express®