Summary
There is a significant unmet clinical need for treatment options for metastatic colorectal cancer (mCRC). This article presents updated overall survival (OS) data from the Patients with Metastatic Colorectal Cancer Treated with Regorafenib or Placebo After Failure of Standard Therapy [CORRECT; NCT01103323] trial. The study demonstrated increased survival benefits following regorafenib treatment in patients with previously treated mCRC.
- Gastrointestinal Cancers
- Oncology Clinical Trials
There is a significant unmet clinical need for treatment options for metastatic colorectal cancer (mCRC). Regorafenib (BAY 73–4506) is an oral multikinase inhibitor that targets multiple tumor pathways [Wilhelm SM et al. Int J Cancer 2011; Mross K et al. Clin Cancer Res 2012; Strumberg D et al. Expert Opin Investig Drugs 2012]. Eric Van Cutsem, MD, University Hospitals Gasthuisberg/Leuven, Leuven, Belgium, presented updated overall survival (OS) data from the Patients with Metastatic Colorectal Cancer Treated with Regorafenib or Placebo After Failure of Standard Therapy [CORRECT; NCT01103323] trial. The study demonstrated increased survival benefits following regorafenib treatment in patients with previously treated mCRC.
CORRECT was a multicenter, randomized, double-blind, placebo-controlled, Phase 3 trial conducted from May 2010 through March 2011 in 114 centers in 16 countries. Patients with mCRC treated with available standard therapies (chemotherapy and monoclonal antibodies) and progressing during or ≤3 months after last standard therapy were randomized to regorafenib (160 mg PO QD, 3 weeks on, 1 week off; no crossover at progression permitted) plus best supportive care (n=505) or placebo (QD, 3 weeks on, 1 week off) plus best supportive care (n=255), with treatment continued until disease progression, unacceptable toxicity, or patient or investigator stopped the treatment. The primary endpoint was OS. Secondary endpoints included disease control rate and safety. This trial met its primary endpoint at a preplanned interim analysis, the results of which have been presented previously [Van Cutsem E et al. J Clin Oncol 2012]. Updated OS data are reported here.
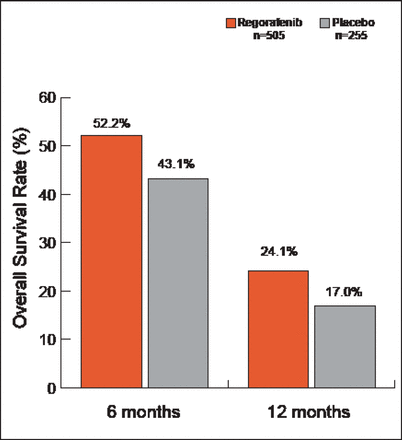
Subjects were mostly white (>77%) with a median age of 61 years (range 22 to 85). For regorafenib and placebo, they had an Eastern Cooperative Oncology Group Performance Status of 0 (52.5% vs 57.3%, respectively) or 1 (47.5% vs 42.7%), and primary disease sites were the colon (64.0% vs 67.5%), rectum (29.9% vs 27.1%), or colon and rectum (5.9% vs 5.5%). The majority of patients in both groups had KRAS mutations, and most had received at least 4 prior lines of therapy. All were previously treated with bevacizumab. The median duration of treatment was 12.1±9.7 and 7.8±5.2 weeks for regorafenib and placebo, respectively. Median OS for this updated analysis (after 566 events) was 6.4 months for regorafenib and 5.0 months for placebo (HR, 0.79; 95% CI, 0.66 to 0.94; p=0.0038). The OS rates at 6 and 12 months were 52.2% and 24.1%, respectively, in the regorafenib arm versus 43.1% and 17.0% in the placebo arm (Figure 1). Disease control rates (partial response + stable disease ≥6 weeks after randomization) were 41.0% versus 14.9% (p<0.000001) in the regorafenib and placebo arms, respectively. With the exception of colon and rectum as the primary site of disease, analysis across all subgroups favored regorafenib.
OS Rates.
More drug-related treatment-emergent adverse events (in particular, hand-foot skin reaction, fatigue, hypertension, diarrhea, and rash and/or desquamation) occurred in the regorafenib arm than in the placebo arm. Subgroup analysis showed few differences in the rate of drug-related adverse events.
The benefits of regorafenib were sustained over time and across prespecified subgroups. Side effects were tolerable and manageable in this patient population.
- © 2012 MD Conference Express®