Summary
This article discusses issues in balancing the treatment of ischemic risk with bleeding associated with anticoagulant and antiplatelet therapies. The TRITON TIMI-38 study evaluated the time dependence of the association of bleeding with mortality in acute coronary syndrome patients undergoing planned percutaneous coronary intervention [Hochholzer W et al. Circulation 2011].
- Coronary Artery Disease
- Thrombotic Disorders
- Interventional Techniques & Devices
TRITON TIMI-38 Study
Elliott Antman, MD, Harvard Medical School, Boston, Massachusetts, USA, discussed issues in balancing the treatment of ischemic risk with bleeding associated with anticoagulant and antiplatelet therapies. The TRITON TIMI-38 study evaluated the time dependence of the association of bleeding with mortality in ACS patients undergoing planned percutaneous coronary intervention (PCI) [Hochholzer W et al. Circulation 2011]. A total of 13,608 patients were treated with aspirin and randomized to clopidogrel (300 mg loading dose/75 mg maintenance dose) or prasugrel (60 mg loading dose/10 mg maintenance dose) for a median duration of 14.5 months.
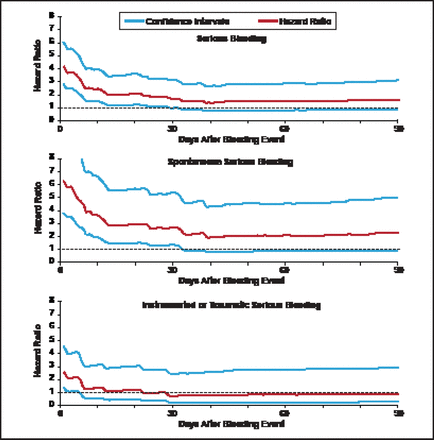
The TRITON TIMI-38 safety cohort consisted of 13,420 patients, excluding patients who experienced coronary artery bypass grafting-related bleeding. Among this cohort, 534 (4%) had a serious bleeding event (TIMI major or minor bleeding). A novel biostatistical approach for the analysis employed a multivariable Cox model including treatment, baseline and procedural variables, and a propensity score for bleeding. Multiple iterative landmark analyses were conducted from Day 1 up to Day 180 after the bleeding event. Observation showed that HRs for serious bleeding, spontaneous serious bleeding, and instrumental or traumatic serious bleeding were initially high but dropped sharply over time (Figure 1). The HR did not differ statistically from baseline risk by 40 days after the bleeding event.
: Hazard Ratios and 95% Confidence Intervals for Bleeding Events.
Hochholzer W et al. Circulation 2012;123(23):2681–2689.
Selection of Therapy
Optimizing therapy for patients with ACS requires careful selection of vascular access site, acute adjunctive pharmacologic therapy, andduration of chronic therapy. The femoral artery has been the traditional catheter entry point; however, radial vascular access is associated with fewer major vascular access complications. Available anticoagulants include unfractionated heparin, enoxaparin, fondaparinux, and bivalirudin. The features of these drugs are shown in Table 1. Both unfractionated heparin and bivalirudin require monitoring of activated partial thromboplastin time and activated clotting time. Bleeding is an issue with all four drugs but may be less with bivalirudin and fondaparinux. Protamine is an antidote for unfractionated heparin and enoxaparin but there is no antidote for fondaparinux and bivalirudin. All four drugs can be used for STEMI and UA/NSTEMI patients.
Anticoagulant Features.
Features of the antiplatelet agents clopidogrel, prasugrel, and ticagrelor are shown in Table 2. All three are administered orally and are associated with bleeding. The effects of ticagrelor are reversible, whereas the effects of clopidogrel and prasugrel are irreversible. Other issues include variable response with clopidogrel, uncertain dosage with prasugrel, and compliance and dyspnea with ticagrelor.
Antiplatelet Features.
Chronic Therapy
The ATLAS ACS 2–TIMI 51 Investigators study evaluated rivaroxaban, a novel anticoagulant administered at low doses in the chronic phase of therapy for ACS [Mega JL et al. N Engl J Med 2012]. A total of 15,526 patients with recent ACS were randomized to twice-daily doses of rivaroxaban 2.5 or 5 mg, or placebo for a mean of 13 months and up to 31 months. The rate of all-cause mortality was reduced with the rivaroxaban 2.5-mg dose (2.9%) compared with placebo (4.5%; HR, 0.68; p=0.002). The ongoing ANTARCTIC trial [NCT01538446] is investigating prasugrel 5-mg fixed dose versus prasugrel adjusted by platelet function test in patients ≥75 years of age undergoing PCI for ACS.
The WOEST trial [NCT00769938] evaluated oral anticoagulant therapy (OAC) plus clopidogrel versus OAC plus clopidogrel and aspirin in 496 patients undergoing PCI with drug-eluting stent (DES) or bare-metal stent (BMS) implantation [Dewilde W et al. ESC 2012; MD Conference Express ESC Highlights 2012]. The results at 12 months showed that the combination of OAC plus clopidogrel versus triple therapy caused less TIMI bleeding (19.5% vs 44.9%; HR, 0.36; 95% CI, 0.26 to 0.50; p<0.001). The ongoing ISAR Triple study [NCT00776633] is investigating 6 weeks of OAC plus clopidogrel and aspirin versus 6 months of OAC plus clopidogrel and aspirin, followed by OAC and aspirin in 600 patients undergoing DES implantation.
The MUSICA-2 trial [NCT01141153] is comparing dual antiplatelet therapy with aspirin, and clopidogrel 75 mg/day with a triple regimen consisting of an OAC, aspirin and clopidogrel in about 300 patients with atrial fibrillation and a low-to-moderate risk of stroke (CHADS ≤2) who are undergoing PCI.
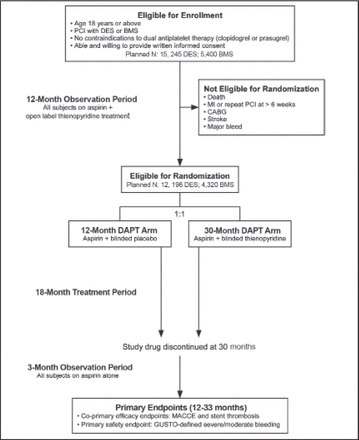
The Dual Antiplatelet Therapy Study [DAPT Study; NCT00977938] is assessing the benefits of 12 versus 30 months of dual antiplatelet therapy versus aspirin for preventing stent thrombosis and major adverse cardiovascular and cerebrovascular events in patients undergoing PCI and stenting [Mauri L et al. Am Heart J 2010]. All patients are receiving open-label dual antiplatelet therapy for 12 months. After 12 months, patients without major adverse cardiovascular and cerebrovascular events or major bleeding will be randomized to placebo or ongoing dual antiplatelet therapy for an additional 18 months. All patients will continue aspirin until study end (Figure 2).
DAPT Study Design.
Reproduced with permission from Mosby, Inc. Mauri L et al. Am Heart J 2012; 160(6): 10 35.
Dr. Antman concluded his presentation by proposing a model of comparative effectiveness research that incorporates the concept of clinical equipoise and embedding randomization in the electronic medical record.
- © 2012 MD Conference Express®