Summary
Responses to oral antiplatelet therapy between patients are variable; thus, bedside assessment has been regarded as an opportunity for individualizing therapy for patients following coronary stent implantation to ensure the optimal platelet inhibition is obtained. The Double Randomization of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation and Interruption Versus Continuation of Double Antiplatelet Therapy [ARCTIC; NCT00827411; Collet JP et al. N Engl J Med 2012] trial evaluated platelet function testing with antiplatelet dose adjustment in suboptimal responders compared with standard of care.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Thrombotic Disorders
Responses to oral antiplatelet therapy between patients are variable; thus, bedside assessment has been regarded as an opportunity for individualizing therapy for patients following coronary stent implantation to ensure the optimal platelet inhibition is obtained. The Double Randomization of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation and Interruption Versus Continuation of Double Antiplatelet Therapy [ARCTIC; NCT00827411; Collet JP et al. N Engl J Med 2012] trial presented by Gilles Montalescot, MD, PhD, Hôpital Pitié-Salpêtrière, Paris, France, evaluated platelet function testing (PFT) with antiplatelet dose adjustment in suboptimal responders compared with standard of care.
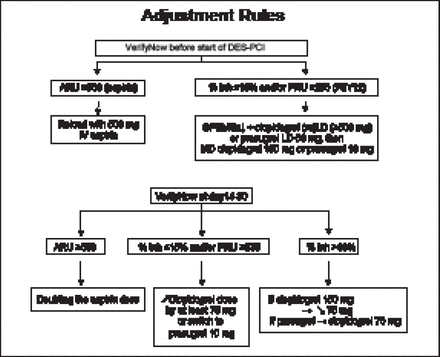
Patients scheduled for planned percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation were randomized to PFT and antiplatelet therapy with dose adjustment for high platelet reactivity (n=1213) versus conventional therapy (n=1227). Patients in both groups then underwent PCI with stent implantation followed by drug and dose adjustment for high platelet reactivity at Day 14 versus conventional therapy. The VerifyNow P2Y12 and aspirin assays were used to estimate the inhibition of platelet aggregation provided by clopidogrel and aspirin. The primary endpoint was death, MI, stroke or transient ischemic attack, stent thrombosis, or urgent revascularization at 12 months. The main secondary endpoints were stent thrombosis or urgent revascularization and major bleeding. The antiplatelet dose adjustment rules used in the study are shown in Figure 1.
Antiplatelet Dose Adjustment Rules.
DES=drug-eluting stent; inh=inhibition; LD=loading dose; MD=maintenance dose; PCI=percutaneous coronary intervention; PRU=platelet reactivity units.
Reproduced with permission from G Montalescot, MD.
Of the total 2440 patients, 20% were women, 37% had a history of diabetes mellitus, and 31% had a history of myocardial infarction (MI). In the PFT group, 7.6% of patients were aspirin poor responders and 35% were thienopyridine poor responders. Among the aspirin poor responders, 85% received on-table aspirin loading. Thienopyridine poor responders received on-table clopidogrel loading (80%), on-table prasugrel loading (3.3%), and on-table GP IIb/IIIa inhibitor loading (80%). Among patients with high on-clopidogrel reactivity at Day 14, 43% had their clopidogrel maintenance increased and 17% were started on prasugrel maintenance dose. Among patients with high on-aspirin reactivity, 46% had their aspirin maintenance dose increased.
At 1 year, the primary endpoint rate was not different between the PFT (34.6%) and conventional therapy groups (31.1%; HR, 1.13; 95% CI, 0.98 to 1.29; p=0.096). The majority of events that comprised the primary endpoint were periprocedural MIs. No significant difference was seen in the 1-year rate of MI between treatment strategy groups (30.3% with PFT-guided therapy vs 28.4% with conventional therapy; HR, 1.08; 95% CI, 0.93 to 1.25; p=0.32), and these neutral findings drove the primary composite results. There also were no significant differences in the rates of the main secondary endpoints (stent thrombosis or urgent revascularization) with PFT-guided therapy compared with conventional therapy (4.9% vs 4.6%; HR, 1.06; 95% CI, 0.74 to 1.52; p=0.77). Data for other ischemic endpoints are shown in Table 1.
Other Ischemic Endpoints.
Key safety outcomes were not significantly different with PFT versus conventional therapy: major bleeding (2.3% vs 3.3%; HR, 0.70; 95% CI, 0.43 to 1.14; p=0.15), minor bleeding (1.0% vs 1.7%; HR, 0.57; 95% CI, 0.28 to 1.16; p=0.12), and major or minor bleeding (3.1% vs 4.5%; HR, 0.69; 95% CI, 0.46 to 1.05; p=0.08).
The ARCTIC study results show that PFT with antiplatelet adjustment before and after stenting does not improve clinical outcomes versus conventional treatment without PFT. These results do not support the routine use of PFT in patients undergoing stenting. The ARCTIC-2 study, in which a second randomization was performed at 1 year after the initial randomization to determine the effect of continuation versus interruption of clopidogrel is ongoing. The Tailored Antiplatelet Therapy Versus Recommended Dose of Prasugrel [ANTARCTIC; NCT01538446] study will evaluate the value of PFT in elderly patients, with a focus on bleeding events. Whether PFT-guided antiplatelet therapy provides benefit for specific types of ischemic events such as spontaneous MI or stent thrombosis is unclear, as the ARCTIC trial was not powered for these individual endpoints and primary findings were largely driven by periprocedural events.
- © 2012 MD Conference Express®