Summary
Relaxin, a naturally occurring peptide, is associated with hemodynamic changes as well anti-ischemic, anti-inflammatory, and antifibrotic effects that may offer benefit to patients with acute heart failure (AHF). This article reports the results of the Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure [RELAX-AHF; NCT00520806] trial designed to test the efficacy and safety of serelaxin, a recombinant version of human relaxin-2, in patients with AHF.
- Heart Failure
- Cardiology Clinical Trials
Relaxin, a naturally occurring peptide, is associated with hemodynamic changes as well anti-ischemic, anti-inflammatory, and antifibrotic effects that may offer benefit to patients with acute heart failure (AHF). John R. Teerlink, MD, University of California, San Francisco, California, USA, reported the results of the Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure [RELAX-AHF; NCT00520806] trial designed to test the efficacy and safety of serelaxin, a recombinant version of human relaxin-2, in patients with AHF. Serelaxin was associated with relief of dyspnea and reduced hospital stay in patients hospitalized with acute decompensated heart failure.
Patients (n=1161) were enrolled in this international, double-blind, placebo-controlled trial who were aged ≥18 years, weighed <160 kg, and were hospitalized for AHF. All patients had dyspnea, congestion on chest radiograph, increased brain natriuretic peptide (BNP) or N-terminal prohormone of BNP (NT-proBNP), mild-to-moderate renal insufficiency, and systolic blood pressure (SBP) >125 mm Hg. Within 16 hours of presentation, eligible subjects were randomly assigned to standard care plus 48-hour intravenous (IV) infusions of placebo (n=580) or serelaxin 30 µg/kg/day (n=581).
The primary endpoints were change from baseline in the visual analog scale area under the curve (VAS AUC) to Day 5 and the proportion of patients with moderate or marked dyspnea improvement measured by Likert scale during the first 24 hours. Subjects had a mean age of 72 years, SBP of 142 mm Hg and evidence of mild-to-moderate renal insufficiency (with estimated glomerular filtration rate 53.5 mL/min/1.73 m2), and average NT-proBNP 5064 ng/L. The mean time to enrollment was 8 hours.
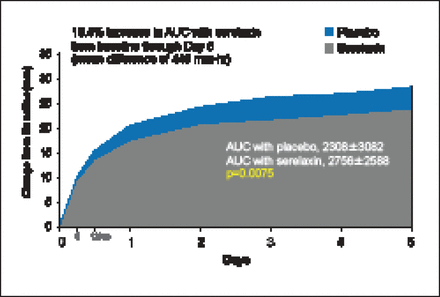
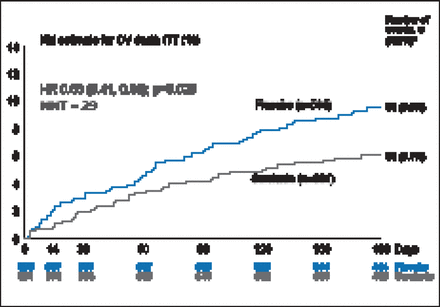
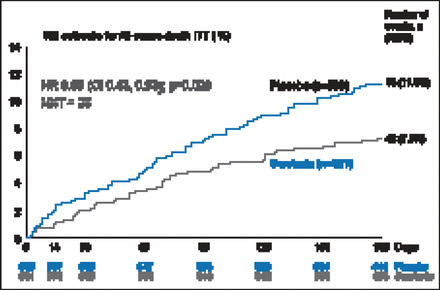
There was a 19.4% improvement in VAS AUC with serelaxin from baseline through Day 5, with a mean difference of 448 mm-hr compared with placebo (p=0.0075; Figure 1). However, there was no statistical difference in relief of dyspnea based on Likert scale measurements taken at 6, 12, and 24 hours. The Kaplan-Meier estimate for the secondary endpoint of time to cardiovascular death or HF/renal function rehospitalization at Day 60 was not significantly different between treatment groups (7.5% vs 6.9%; HR, 1.08; 95% CI, 0.70 to 1.66; p=0.73). There was, however, a significant decrease in the exploratory endpoint of cardiovascular death through Day 180 with serelaxin as compared with placebo (9.6% vs 6.1%; HR, 0.63; 95% CI, 0.41 to 0.96; p=0.028; Figure 2) as well as a significant 37% reduction in all-cause death (Figure 3).
Dyspnea Relief (VAS AUC).
AUC=area under the curve; VAS=visual analog scale.
Cardiovascular Death Through Day 180.
CV=cardiovascular; ITT=intention-to-treat; KM=Kaplan-Meier; NNT=number needed to treat.
All-Cause Death Through Day 180.
KM=Kaplan-Meier; ITT=intention-to-treat; NNT=number needed to treat.
Figures 1 through 3 reprinted from The Lancet [Epub ahead of print Nov. 7, 2012], Teerlink JR et al. Serelaxin, Recombinant Human Relaxin-2, for Treatment of Acute Heart Failure (RELAX-AHF): A Randomised, Placebo-Controlled Trial.
Copyright 2012, with permission from Elsevier.
Treatment with serelaxin was associated with improvements in the signs and symptoms of congestion at Day 2 as well as biomarkers of neurohormonal activation and myocyte stress. Use of seralaxin was also associated with reductions in resource utilization including intravenous diuretics and length of ICU stay and hospital (p<0.05).
Adverse events (AEs), including serious AEs, were similar between treatment arms except for renal impairment-related AEs, which occurred significantly (6% vs 9%; p=0.03) less often in the serelaxin arm. Dr. Teerlink concluded that the findings of the RELAX-HF trial suggest some benefit to early treatment with serelaxin in patients with AHF but additional large clinical outcomes studies are needed to further define its role in the management of AHF, the optimal target population, and the cost-effectiveness of therapy [Teerlink JR et al. Lancet 2012; Metra M et al. J Am Coll Cardiol. In press].
- © 2012 MD Conference Express®