Summary
Results from two Phase 2 trials indicate that intravenous infusions of RN316, a humanized IgG2?a monoclonal antibody that inhibits proprotein convertase subtilisin kexin type 9 (PCSK9), significantly lowers low-density lipoprotein cholesterol in hypercholesterolemic subjects already on high to maximal doses of statins.
- Cardiology Clinical Trials
- Lipid Disorders
Results from two Phase 2 trials reported by Barry Gumbiner, MD, Pfizer, Inc., South San Francisco, California, USA, indicate that intravenous infusions of RN316, a humanized IgG2Δa monoclonal antibody that inhibits proprotein convertase subtilisin kexin type 9 (PCSK9), significantly lowers low-density lipoprotein cholesterol (LDL-C) in hypercholesterolemic subjects already on high to maximal doses of statins.
Although statins are first-line therapy for reducing LDL-C and cardiovascular events, many patients are unable to achieve LDL-C goals or tolerate high doses of statin medication. PCSK9, a protein that reduces the number of LDL receptors, leading to diminished hepatic clearance capacity for plasma LDL-C and increased LDL-C levels, is a new therapeutic target for LDL-C lowering. RN316 binds to PCSK9, preventing PCSK9-mediated down-regulation of the LDL receptor, thereby improving LDL-C clearance and reducing LDL-C levels.
Two Phase 2, randomized, double-blind, placebo-controlled, 12-week studies were conducted to assess the efficacy, safety, and tolerability of RN316 when added to high [A Multiple Dose Study of PF-04950615 (RN316) in Subjects on High Doses of Statins; NCT01342211] or maximum [A Multiple Dose Study of PF-04950615 (RN316) In Subjects On Maximum Doses of Statins; NCT01350141] doses of statins (atorvastatin, rosuvastatin, and simvastatin) in subjects with primary hypercholesterolemia. Subjects were eligible if they had an LDL-C ≥100 mgdL in the high-dose statin study and ≥80 mg/dL in the maximal-dose statin study. Subjects in the high-dose statin study were randomized to receive 0.25, 1.0, 3.0, or 6.0 mg/kg doses of intravenous (IV) RN316 or placebo every 4 weeks, while those in the maximal-dose statin study were randomized to 1.0 or 3.0 mg/kg of RN316 or placebo. RN316 dosing was interrupted if LDL-C reached ≤25 mg/dL and resumed once it reached ≥40 mg/dL at a later visit. Subjects were on average aged 55 to 61 years, and overweight or obese with an average body mass index of 30 kg/m2.
Across the 5 arms, mean baseline LDL-C was ∼123 mg/dL, total cholesterol (TC) was ∼197 mg/dL, and HDL-C was ∼49 mg/dL. Triglycerides ranged from 115 mg/dL to 173 mg/dL. The primary endpoint was the mean percent change in lipid levels from baseline to Week 12.
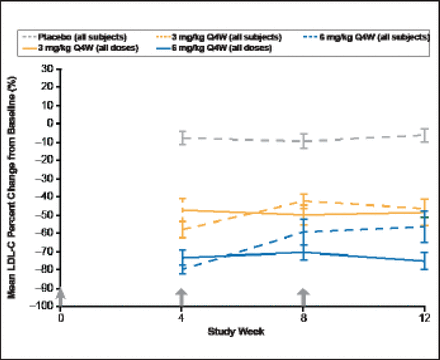
Data were pooled from the 2 studies (n=135) for this analysis. LDL-C and TC decreased <15% in the placebo, and 0.25 mg/kg and 1 mg/kg RN316-dose groups, with reductions in LDL-C of 46% and 56% with 3- and 6-mg/kg doses of RN316, respectively (p<0.001). For the 3- and 6-mg/kg doses of RN316, there were also significant reductions in TC (30% and 37%, respectively [p<0.001]) and significant increases in HDL-C (7% and 11%, respectively [p<0.05]; Figure 1). There was no significant change in triglyceride levels. More than half of the subjects in the 3- and 6-mg/kg dose groups had at least 1 interrupted dose (19 [59.4%] and 12 [70.6%], respectively). Without dose interruption, overall LDL-C lowering at Week 12 would have been similar to maximal LDL-C lowering observed at Week 4, as confirmed by the sustained LDL-C lowering of 75% in the 6-mg/kg dose subgroup that had no dose interruption.
Mean LDL-C Percent Change from Baseline.
Values are mean ± SE; B1481005 and B1481012 data combined, modified ITT results; results include subjects who had dosing interrupted for LDL-C ≤25 mg/dL; * p<0.05; **p<0.001
Reproduced with permission from B Gumbiner, MD.
Approximately two thirds of all subjects experienced adverse events (AEs) that were mild in nature and resolved without intervention; Dr. Gumbiner said that majority these were reported by the investigators as not likely to be related to the study drug. Overall AEs were balanced between the randomization groups. The development of antidrug antibodies (non-neutralizing) occurred in 5% of subjects receiving RN316, but there were no hypersensitivity reactions. Dr. Gumbiner concluded that overall RN316 appeared to be efficacious, safe, and well tolerated at the doses studied.
- © 2012 MD Conference Express®