Summary
Postoperative atrial fibrillation (AF) flutter occurs in approximately 1 of 3 patients undergoing cardiac surgery [Hogue CW Jr et al. Chest 2005; Mitchell LB et al. Can J Cardiol 2011], generating a need for new therapies to prevent it and its associated morbidity and healthcare costs [Mozaffarian D et al. JAMA 2012]. This article discusses findings from the Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation trial [OPERA; NCT00970489].
- Arrhythmias
- Cardiology Clinical Trials
Postoperative atrial fibrillation (AF) flutter occurs in approximately 1 of 3 patients undergoing cardiac surgery [Hogue CW Jr et al. Chest 2005; Mitchell LB et al. Can J Cardiol 2011], generating a need for new therapies to prevent it and its associated morbidity and healthcare costs [Mozaffarian D et al. JAMA 2012]. Roberto Marchioli, MD, MPH, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy, reported findings from the Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation trial [OPERA; NCT00970489], which were simultaneously published in the Journal of the American Medical Association online [Mozaffarian D et al. JAMA 2012].
A few small trials have evaluated whether long-chain n-3 polyunsaturated fatty acids (PUFAs) reduce postoperative AF, with mixed results. The purpose of OPERA, a large multinational, randomized, double-blind, placebo-controlled clinical trial, was to examine whether perioperative intake of n-3 PUFAs would reduce the occurrence of postoperative AF in cardiac surgery patients aged ≥18 years scheduled for cardiac surgery on the following day or later who had sinus rhythm on screening electrocardiogram (ECG).
The primary endpoint was any postoperative AF >30 seconds duration confirmed by rhythm strip or 12-lead ECG. Secondary endpoints were postoperative AF lasting longer than 1 hour resulting in symptoms or treated with cardioversion; postoperative AF, excluding atrial flutter; time to first postoperative AF; number of AF episodes per patient; hospital utilization; and major adverse cardiovascular events, 30-day mortality, bleeding, and other adverse events [Mozaffarian D et al. JAMA 2012].
A total of 1516 patients undergoing cardiac surgery in 28 centers in the United States, Italy, and Argentina were randomized to receive fish oil (1 g capsules containing ≥840 mg n-3 PUFAs as ethyl esters) or placebo, with preoperative loading of 10 g over 3 to 5 days (or 8 g over 2 days) followed postoperatively by 2 g/day until hospital discharge or postoperative Day 10, whichever came first.
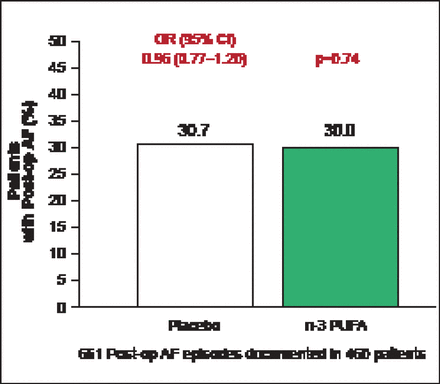
The average age of enrolled patients was 64 years; 72.2% were men and 51.8% had planned valvular surgery. The primary endpoint occurred in 233 (30.7%) patients assigned to placebo versus 227 (30.0%) assigned to n-3 PU FA s (OR, 0.96; 95% CI, 0.77 to 1.20; p=0.74; Figure 1). None of the secondary endpoints were significantly different between the placebo and fish oil groups, including postoperative AF that was sustained, symptomatic, or treated (231 [30.5%] vs 224 [29.6%]; p=0.70) or number of postoperative AF episodes per patient (1 episode: 156 [20.6%] vs 157 [20.7%]; 2 episodes: 59 [7.8%] vs 49 [6.5%]; or ≥3 episodes: 18 [2.4%] vs 21 [2.8%]; p=0.73).
Other secondary endpoints were not significant: postoperative AF excluding atrial flutter (p=0.87), total number of days with any postoperative AF (p=0.58), and proportion of days free of postoperative AF (p=0.882).
OPERA Primary Endpoint: Postoperative AF Episodes.
Reproduced with permission from R Marchioli, MD, MPH, and D Mozaffarian, MD, PhD.
Based on the data, Prof. Marchioli concluded that postoperative AF remains an enigmatic and difficult-to-prevent complication of cardiac surgery. While n-3 PUFA appeared to be safe and well-tolerated with no evidence of increased bleeding, the OPERA trial “provides evidence that perioperative n-3 PUFA does not appreciably reduce postoperative AF in the acute setting of cardiac surgery.”
- © 2012 MD Conference Express®