Summary
Odanacatib is a selective, potent, and reversible cathepsin K inhibitor being developed for the treatment of postmenopausal osteoporosis. This article discusses results of A Study to Evaluate the Safety, Tolerability, and Efficacy of Odanacatib (MK0822) in Postmenopausal Women Previously Treated with Alendronate. The trial found odanacatib to be safe and effective for increasing bone mineral density in postmenopausal women previously treated with alendronate.
- Rheumatology Clinical Trials
- Metabolic Bone Disease
Odanacatib is a selective, potent, and reversible cathepsin K inhibitor being developed for the treatment of postmenopausal osteoporosis. Roland Chapurlat, MD, Hôpital Edouard Herriot, Lyon, France, reported results of A Study to Evaluate the Safety, Tolerability, and Efficacy of Odanacatib (MK0822) in Postmenopausal Women Previously Treated with Alendronate. The trial found odanacatib to be safe and effective for increasing bone mineral density (BMD) in postmenopausal women previously treated with alendronate.
Unlike denosumab and bisphosphonates, which reduce both bone resorption and bone formation, odanacatib inhibits bone resorption while preserving bone formation (to some extent) by limiting the digestion of the collagen bone matrix while sparing osteoclastic activity and function [Leung P et al. Bone 2011; Duong LT. Bonekey Reports 2012].
This randomized, double-blind, placebo-controlled, 24-month study evaluated the effect of odanacatib on BMD in postmenopausal patients previously treated with alendronate for ≥3 years (mean 5.5 years). Postmenopausal women aged ≥60 years (n=246; mean age 71 years and mostly white) with no previous hip fractures and BMD T-scores at any hip site between <−2.5 and >−3.5 in patients without a history of fracture (<1.5 to >−3.5 in patients with a history of fracture) received odanacatib 50 mg or placebo QW, along with vitamin D3 5600 IU and open-label calcium supplementation to ensure a total daily intake of 1200 mg. The primary study endpoint was BMD at the femoral neck with odanacatib versus placebo at Month 24. The key secondary endpoint was BMD at the femoral neck with odanacatib compared with baseline at Month 24. Other secondary endpoints were BMD at the trochanter, total hip, lumbar spine, 1/3 radius, biochemical markers of bone resorption and formation, and safety and tolerability.
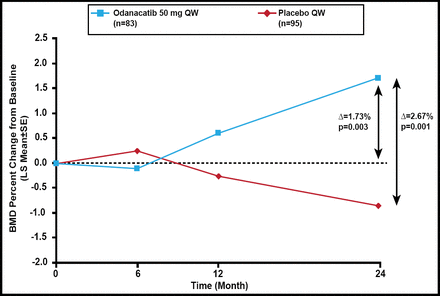
Baseline characteristics were balanced between the 2 groups. At 24 months, the percent change in femoral neck BMD was greater in odanacatib-treated patients compared with placebo (Δ=2.67%; p<0.001) and baseline (Δ=1.73%; p=0.003; Figure 1). Similar significant results for total hip, trochanter, and lumbar spine BMD were noted. There was no difference in 1/3 radius BMD between odanacatib and placebo.
Primary Endpoint: Femoral Neck BMD.
BMD=bone mineral density; LS=least squares; QW=once weekly..
Reproduced with permission from R Chapurlat, MD.
Odanacatib further decreased bone resorption and increased bone formation in this population of patients on long-term treatment with alendronate as suggested by the continued decreases in the urine N-telopeptide/creatinine ratio (p<0.001) and increase in serum N-terminal propeptide of type 1 collagen values (p=0.011).
Odanacatib was safe and well tolerated. The most common adverse events, which occurred at a similar rate in both groups, were back pain, arthralgia, and urinary tract and respiratory infections. The authors concluded that odanacatib may offer a viable alternative for patients who are in need of continued therapy and want to obtain benefit beyond that already provided from alendronate.
- © 2012 MD Conference Express®