Summary
Strontium ranelate, an antiosteoporotic drug developed for the treatment of postmenopausal osteoporosis, delayed radiographic progression of knee osteoarthritis and improved symptoms. This reports on The Efficacy and Safety of Two Doses of Strontium Ranelate Versus Placebo, Administered Orally for Three Years in the Treatment of Knee Osteoarthritis [SEKOIA] trial [Reginster JY et al. Ann Rheum Dis 2012].
- Metabolic Bone Disease
- Metabolic Bone Disease Clinical Trials
Strontium ranelate (SrRan), an antiosteoporotic drug developed for the treatment of postmenopausal osteoporosis, delayed radiographic progression of knee osteoarthritis and improved symptoms, reported Jean-Yves Reginster, MD, PhD, Université of Liège, Liège, Belgium, in a late-breaking clinical trial [Reginster JY et al. Ann Rheum Dis 2012].
Osteoarthritis affects more than 50% of the elderly. Most treatments focus on symptoms and are not disease modifying. SrRan was shown to stimulate cartilage matrix formation in vitro and to reduce radiographic spinal osteoarthritis progression in osteoporotic women. The objective of The Efficacy and Safety of Two Doses of Strontium Ranelate Versus Placebo, Administered Orally for Three Years in the Treatment of Knee Osteoarthritis [SEKOIA] trial was to compare the efficacy and safety of 1 g QD and 2 g QD of SrRan versus placebo for reducing radiological progression of knee osteoarthritis.
This was a Phase 3, international, double-blind, placebo-controlled, 3-year study. The 2-g dose is the current marketed dose for the management of osteoporosis. Ambulatory white men and women (n=1683) aged ≥50 years with primary knee osteoarthritis based on the clinical criteria of the American College of Rheumatology [Altman R et al. Arthritis Rheum 1986] were included in the study. Radiologic criteria included Kellgren and Lawrence Grade II or III, arthritis predominant in the medial compartment of the knee, and joint space width between 2.5 and 5 mm. The primary study endpoint was the radiological joint space narrowing (JSN) of the medial tibio-femoral compartment of the target joint. Other endpoints included radiological and radioclinical progression, and pain and function assessment. The patient population was consistent with the selection criteria and osteoarthritis population in general. There were no relevant between-group differences in demographic and disease characteristics. The dropout rate was 42%.
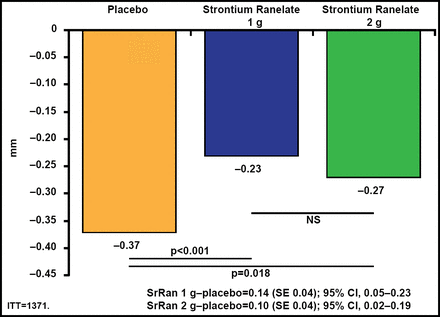
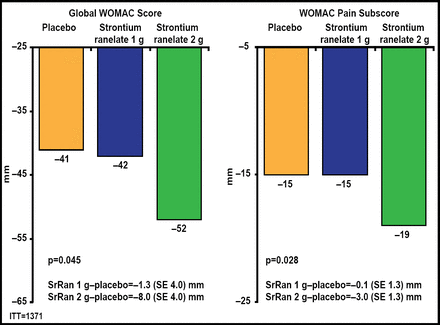
Both doses of SrRan significantly (p<0.02) lowered JSN compared with placebo (Figure 1). There were also fewer radiological and radioclinical progressors in both SrRan dose groups compared with the placebo group. Greater reductions in global Western Ontario and McMaster Universities Arthritis (WOMAC) scores (p=0.045) and pain (p=0.028) subscores were observed with 2 g QD of SrRan (Figure 2). A significantly (p<0.03) greater number of patients in the 2-g/day SrRan group reached the minimally perceptible clinical improvement and minimal clinically important improvement thresholds compared with patients in the placebo group. SrRan was well tolerated with adverse events and serious adverse events occurring similarly in each dose groups and placebo group.
Significantly Lower JSN in Both Strontium Ranelate Groups Compared with Placebo.
ITT=intention-to-treat; JSN=joing space narrowing; SE=standard error; SrRan=strontium ranelate. Adapted from Reginster JY et al. Efficacy and Safety of Strontium Ranelate in the Treatment of Knee Osteoarthritis: Results of a Double-Blind, Randomised Placebo-Controlled Trial. Ann Rheum Dis 2012 Nov 9 [Epub ahead of print].
SrRan 2 g Improves Symptoms Compared with Placebo.
SrRan=strontium ranelate; WOMAC=Western Ontario and McMaster Universities Arthritis Index. Adapted from Reginster JY et al. Efficacy and Safety of Strontium Ranelate in the Treatment of Knee Osteoarthritis: Results of a Double-Blind, Randomised Placebo-Controlled Trial. Ann Rheum Dis 2012 Nov 9 [Epub ahead of print].
The structure-modifying effect of SrRan is translated clinically into a lower number of patients having a radiological progression over thresholds known to be predictive of osteoarthritis-related surgery. This suggests that SrRan could reduce the number of patients needing knee surgery in the long-term.
- © 2012 MD Conference Express®